Soumendu Majumdar
Last Activity: 5 Years ago
Dear Dushyant,
Question-5:a)
Let us use the following chemical equation: A ---> products.
The decrease in the concentration of A over time can be written as: - d[A] / dt = k [A]
Rearrangement yields the following: d[A] / [A] = - k dt
Integrate the equation, which yields: ln [A] = - kt + C
Evaluate the value of C (the constant of integration) by using boundry conditions. Specifically, when t = 0, [A] = [A]o. [A]o is the original starting concentration of A.
Substituting into the equation, we obtain: ln [A]o = - k (0) + C.
Therefore, C = ln [A]o
We now can write the integrated form for first-order kinetics, as follows:
ln [A] = - kt + ln [A]o
This last equation can be rearranged into several formats, such as:
ln ([A] / [A]o) = - kt
[A] / [A]o = 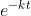
[A] = [A]o 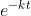
Question-6:a) If [A]0 be initial concentration and k be rate constant then using above formula
[A] = [A]o 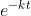
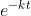 = 0.8 {For 20% completion, concentration of reactants is [A]0 – (0.2)[A]0}
= 0.8 {For 20% completion, concentration of reactants is [A]0 – (0.2)[A]0}
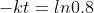
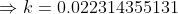 (You can round off the value as per your convenience)
(You can round off the value as per your convenience)
Hence for 75% completion
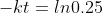
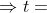
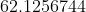
So time taken for 75% completion is 62.126 minutes(approx).
Question-6:b) t1/2 is called half-life of the radioactive substance which is the time taken for its concentration to become half of its initial concentration.
Using above formula you will get
t1/2 = 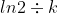
Also for 99.9% completion time taken is
t = 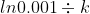
Hence their ratio is given by (or relation between them is)
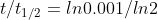
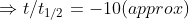
Hope it helps!
regards,
Soumendu





