Drago’s rule is basically a rule of hybridisation.
Conditions for dragos rule:
i.) no. of sigma bonds+ l.p.=4
ii.) the central atom must belong to 3rd, 4th, 5th or 6th group(not to 2nd)
iii.) the electronegativity of central atom must be
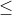
2.5
iv.) Atleast 1 lone pair must be there on central atom.
if these conditions are fulfilled then the compound follows dragos rule, which says that there will be bo need to consider hybridisation.
drago’s suggested an empirical formula, which said that, in these cases, the bond angle is exceptionally small approx. 90~94 degree.And this is the reason why PH3 has an exception bond angle of 93.5 degree.




