Aarti Gupta
Last Activity: 10 Years ago
Neils Bohr proposed new atomic model in order to overcome the shortcomings of Rutherford’s model.The
postulates of Bohr atomic model are as follows:-
1).The electron in an atom revolves around the nucleus only in certain selected circular orbits,which are called as
energy shells or energy levels.These orbitals are associated with definite energies and numbered as
1,2,3,4.... etc,and designated as
K,L,M,N.... etc.shells.
2.Only those orbits are permitted in which the angular momentum of the electron is a whole no.multiple of h/2
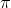
i.e
mvr = n h/2pi, whwere n = 1,2,3...n
m = mass of e-
v = velocity of e- and
r = radius of orbit
In other words
angular momentum of electrons in an atom is quantised.3.As long as an e- remains in a particular orbit,it does not lose or gain energy,which means that energy of an e- in a particular orbit remains constant and so these orbits are also called
stationary states.
4.When an energy from an external source is supplied to the e-,it may jump to
some higher energy level by absorbing a definite amount of energy and when it jumps back to the lower energy level it radiates same amount of energy in the form of a photon of radiation such that,
deltaE = E2 – E1 = h
where

is the frequency of the radiation emitted when e- jump from the energy level having energy E2 to the energy level having energy E1.



