Aman Bansal
Last Activity: 12 Years ago
Dear Mani,
C2H5NH2 + HNO2 -> C2H5OH + H2O + N2 (Ethyl amine)
The Hofmann degradation is a reaction between an amide and a mixture of bromine and sodium hydroxide solution. Heat is needed.
The net effect of the reaction is a loss of the -CO- part of the amide group. You get a primary amine with one less carbon atom than the original amide had.
The general case would be (as a flow scheme):

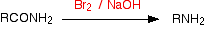
If you started with ethanamide, you would get methylamine. The full equation for the reaction is:
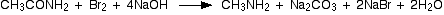
The Hofmann degradation is used as a way of cutting a single carbon atom out of a chain.
Cracking IIT just got more exciting,It s not just all about getting assistance from IITians, alongside Target Achievement and Rewards play an important role. ASKIITIANS has it all for you, wherein you get assistance only from IITians for your preparation and win by answering queries in the discussion forums.
https://www.askiitians.com/packages/packages.aspx
So start the brain storming…. become a leader with Elite Expert League ASKIITIANS
Thanks
Aman Bansal
Askiitian Expert











