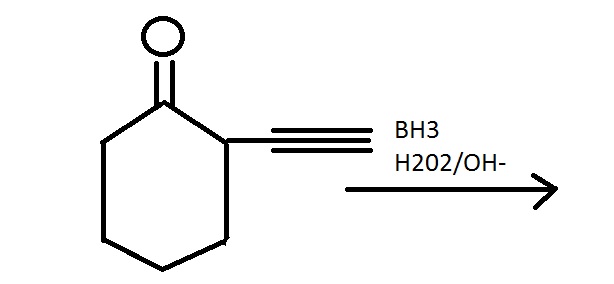
In the above reaction, Markonikoff product i.e the ketone is more favoured than the expected antimarkonikoff product , the aldehyde. Can anyone pls explain me why ??? Probably one factor is stability of enolic form of ketone by hydrogen bonding. But what r the other reasons...










