Common Mistakes in Exams “Galtiyaaa bhi kabhi achhiii hoti hai…” :)
A bright sunny day, time nearly 12:30 PM and coming out of the examination hall, walking along the roadside and discussing what the hell was there in the exam..
Two students shouting, “R u people damn kidding??? Why are you discussing the paper??†The another couple of students saying, “Its okk, let us discuss the questions so that in future to baachh jaaye..â€Â
And one after the other there comes a series of questions where you end up saying..Shit man..this also went wrong..
Sooooooooo….I better thought why shouldn’t I tell you those common questions so that you people would say, “Thanks to the day u read this article!!!!â€Â
Aaaj mauka bhi hai aur dastoor bhi, why not learn something from my mistakes..Bohat exams diye hai yaar!!
 “Class 11th Pre Boards Question 2009â€Â
You know what ‘Bade bade shehro mei chotti chotti galtiyaaan to hoti rehti hai’..and one of the chotti galti that can cause a blunder in your exam is taking the atomic weight of Hydrogen as 2
That is quite obvious, “ Atomic number of Lithium =3, Helium =2, hydrogen =1â€Â so mass numbers should be.. Lithium =6, Helium =4 and hydrogen =2..
Arrreeeeyyyyyy…
This is not a sequence and series ka question right..
Hydrogen has atomic number 1 and mass number also 1..
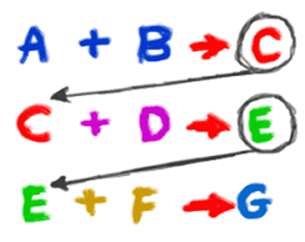
Let me ask you a series of questions..
1+1= ?
2+2= ?
2+3= ?
Many of you are gonna give the answers as…2,4,6…
Look into the answers again..
2+3 = 6 ???
Achhhaaaa???
See this is the another common mistake we generally commit in our exams..
are different…
Let me take another situation now,
All of you might have heard this famous dialogue “Kitne aadmi the kaaliya???†from the movie Sholay..
Okkk, on the similar lines let me ask you a question..
There is a beaker containing 36 mL of water, 32 mL of oxygen and 28 mL of nitrogen..The volume of the container is 2L, and the temperature is 273 K. Can you tell me what is the pressure of the container in terms of R itself…
Fine take 3 minutes and solve the question..
I can bet the answer you people would have got is..
“Using the ideal gas equation, PV=nRT
Let’s first find n..
n = total number of moles
= (36/18) + (32/32) + (28/28)
=4 moles..
Ans will be..
P= 4*R*273/2L = 546 R
R u sure this is the right technique and I have not done anything wrong in the question?
Dear readers…Remember here the total number of moles won’t be 4 rather it will be two only..
Ideal gas equation is only for gases na….not for liquids and solids…
“Waqt ki nazaaqat aur ghadii ko samajhna is zaroorii..â€Â
Let me now give you another common mistake which you people do in exams. So let’s welcome another stupid mistake from another good chapter, redox reaction
What is the n factor of hypo (Na2S2O3) in the reaction?
Hypo + KMnO4……..??????
May be half of you will give the answer as8 but the other half of you would have answered as 1..
Why so???
This is because we all learnt.. “Hypo ka n factor is 1..and where did u learn that??
Yaar NCERT mei given thaâ€Â
Students, I do agree NCERT said n factor was 1 but that was for only its reaction with iodine and not always..
Do remember, here first you need to write down the reaction and go by the traditional method of finding out the change in oxidation number of the reactants and products..
Na2S2O3 + KMnO4 + H2O = K2SO4 + KOH + MnO2 + Na2SO4
You will find the change in oxidation number from 2 to 6 and so the overall change will be (4*2 = 8 and not 1)
Tip: Reaction likhni chaahiyyeee….
Okkkkk…Now let me share one another mistake..
You have heard right..
“Life mei pyaar ek hi baar hota hai, shaaadi bhi..and vo bhi bas ek se hi..â€Â
U remember a very common chapter from organic chemistry, General Organic Chemistry, RS Nomenclature of chiral carbon..
You are always told, to decide the priority order follow the CIP rules…And dear students, don’t messsssssssuppppppp with the concept…You were always told to compare the mass of the first atom attached to the chiral carbon and not the entire group attached to it..
Here N will always be given first priority and not COOH..
N has more atomic mass as compared to carbon..
Now please don’t do COOH is bulky and heavier than NH2..
So readers, I think these are certain common mistakes which we as students commit in our exams. These mistakes appear quite simple and many of you might be thinking, “Weee…No No No..Wecan’t do such stupid mistakes in our exams…â€Â
But dear readers, being a tutor, I have many a times seen students committing such mistakes once or twice in their exams..
We don’t commit such mistakes in easy questions..We generally do such stupidity in lengthy 4 or 6 marks questions, when we are so much lost in focusing on the important and difficult part of the question that we loose the track of simpler stuff…â€Â
I have shared you the feelings of many good and bright students who have committed such illogical mistakes in their exam which led to a blunder..
Now, Let me keep all the humor stuff aside..
Just gonna tell you a small trick for your exams..
“Take care of small things and the big things will ultimately fall into place.â€Â
“Aaap humari galtiyo se sikhiyyeee, hum fir millenge chalte chalte..â€Â
I think you all are getting the feel as if again and again I am saying, “Mei hu naâ€Â. Ab who actually I am and why should you be learning from my mistakes..
Now this is the time when I tell you who actually I am..
Heyyy readers, I am Sakshi Ganotra, a passout of IIT Roorkee, and a chemistry faculty at askIITians. Waise to know more about me and my video lectures (I recorder Ionic Equilibrium) for you people, you can refer to these links..
Prepared by: Sakshi Ganotra Find online Study Packages
- Excited
- Fascinated
- Amused
- Bored
- Sad
- Angry