Top 5 crystalline structure and their scientific explanation
Quite often we come across the term “SOLIDSâ€Â. It undoubtedly is the most commonly used state of matter.
But, have you ever thought what makes a substance solid??
Or how many types of solids do we have??
Why not all solids behave in the same manner??
No…right?? So today through this column we will learn these answers.
 Now we know that solids can exist in variety of forms be it in the form of rocks, diamonds, fur or even talc. But the only common physical property underlying in them is strong forces of attraction.
Now we know that solids can exist in variety of forms be it in the form of rocks, diamonds, fur or even talc. But the only common physical property underlying in them is strong forces of attraction.
These variety of solids can be basically categorized into two types: – “Amorphous and Crystallineâ€Â
Amorphous means “of no shapeâ€Â. These are those solids in which the building constituents are arranged in a haphazard manner instead of regular. As a result, they do not possess any characteristic shape or sharp melting point. For instance, glass, rubber, plastics etc.
 Do you know that amorphous substances are not true solids rather they exist as an intermediate state between solid and liquid. Therefore they are also referred as Pseudo solids or super cooled liquids and have the tendency to flow.
Do you know that amorphous substances are not true solids rather they exist as an intermediate state between solid and liquid. Therefore they are also referred as Pseudo solids or super cooled liquids and have the tendency to flow.
Crystal on the other hand, is defined as a solid object having definite geometry with sharp edges and flat faces. Their constituent particles are arranged in a regular manner throughout the three-dimensional network.
You might have seen many common crystals in the form of rocks and minerals like NaCl (rock salt), Diamond, CsCl, Ag, quartz etc. But do you how these are formed??
So now let’s have an exciting tour regarding their structures !!
 All crystals are polyhedral consisting of regularly repeating arrays of atoms, ions or molecules. The geometrical form consisting of only the regular array of points in space is known as space lattice or crystal lattice.
All crystals are polyhedral consisting of regularly repeating arrays of atoms, ions or molecules. The geometrical form consisting of only the regular array of points in space is known as space lattice or crystal lattice.
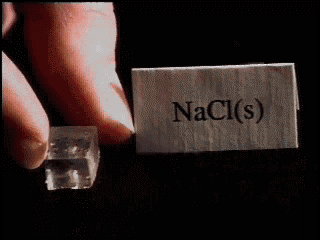
NaCl STRUCTURE :-
Though NaCl or salt looks like this,
But if you keenly observe in laboratory then you will find that the arrangement of Na+ ions and Cl- ions are somewhat like this.
 Here you can see that Chlorine atoms occupy corner as well as face center position whereas sodium atoms occupy edge center and body center of the cube.
Here you can see that Chlorine atoms occupy corner as well as face center position whereas sodium atoms occupy edge center and body center of the cube.
Imagine more of similar crystals in 3-D and you will find that, each chlorine atom is attached to 6 sodium atoms whereas each sodium atom is attached to 6 chlorine atoms. This is known as “COORDINATION NUMBERâ€Â
DIAMOND STRUCTURE :-
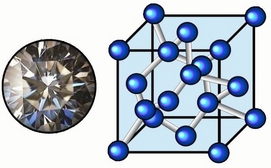 The ever so glittery diamond has a complicated structure. In this Carbon atoms lie at the corners, face centres and at body diagnols (2 each on 1 diagnol) of the cube.
The ever so glittery diamond has a complicated structure. In this Carbon atoms lie at the corners, face centres and at body diagnols (2 each on 1 diagnol) of the cube.
Isn’t it weird?? I know it is…And do you know that the coordination number of diamond is 4. (Come on, each C is attached to 4 more carbons only…it’s tetravalent after all!!).
Ag CRYSTAL STRUCTURE:-
 Silver jewelry looks too pretty but ask a chemist, and they will say that “I wish their structure would have been pretty enoughâ€Â
Silver jewelry looks too pretty but ask a chemist, and they will say that “I wish their structure would have been pretty enoughâ€Â
In silver, the three layers of silver atoms are arranged in this manner. This kind of stacking of atoms is known as CCP (Cubic close packing). As you can see, each sphere (it will be easy to understand if you will take the central atom of hexagon in this structure) touches six others in the same plane, three in the plane above and three in the plane below.
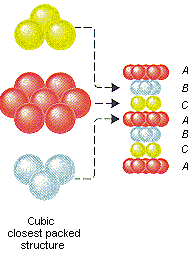 I guess it’s quiet evident that this makes the coordination number of silver as 12!!
I guess it’s quiet evident that this makes the coordination number of silver as 12!!
So, that was all about the crystals. To learn more you can visit – Solid State.
If you have any query kindly post here:
ÂÂ
ÂÂ
Submitted by
Bhavya Agarwal
Bhavya Agarwal has completed Master of Science degree from IIT Delhi. She has worked on “Cisplatin as Penicillin of Cancer. Cisplatin is a chemotherapy drug used to treat cancers & tumors.
- Excited
- Fascinated
- Amused
- Bored
- Sad
- Angry

