Exemplars of Hybridization in Chemistry
Hybridization is a very frequently used word of chemistry. Though in simple words it means intermixing, but the exact definition of Hybridization is as follows:
Hybridization is the mixing of two orbital molecules to form new orbital molecules. This is basically aimed at offering maximum stability to the molecule. There can be a variety of hybridization’s like:
- sp
- sp2
- sp3
- sp3d
- sp3d2
- sp3d3
We list here some examples of sp hybridization in detail:
1)   Beryllium Chloride (BeCl2)
‘Be’ has electronic configuration as 1s2 2s2 in the ground state. Due to the absence of any unassociated electron, excitation takes place fostering its one 2s electron into the empty 2p orbital. Thus, in this phase, the electronic configuration is 1s2 2s1 2p1.
BeCl2 is linear in shape. In case the beryllium atom formed bonds using pure orbitals, the shape of the molecule might have been angular. Hence for this reason, the below given sp hybridization was proposed.
During the sate of excitation, the atom undergoes sp hybridization involving the mixing of 2s and 2p orbitals. Thus two half filled ‘sp’ hybrid orbitals are formed, which are arranged linearly. These half filled Sp-orbitals form two ÃÆ’ bonds with two ‘Cl’ atoms and hence this leads to the linear shape of Thus BeCl2 with the bond angle of 180o.
2)   Acetylene (C2H2)
The ground state configuration of ‘C’ being 1s2 2s2 2px12py1 has only two unassociated electrons. But carbon makes four bonds since its valency is four. For this 4 unpaired electrons are required. Hence it promotes its 2s electron into the empty 2pz orbital in the excited state.
Thus in the excited state, the electronic configuration of carbon is 1s2 2s1       2px12py12pz1. Each carbon atom undergoes ‘sp’ hybridization by using a 2s and one 2p orbitals in the excited state to give two half filled ‘sp’ orbitals, which are arranged linearly.
By the use of Sp-orbitals, a ÃÆ’sp-sp bond is formed between the two carbon atoms. At the same time there are two unhybridized p orbitals i.e., 2py and 2pz on each carbon atom which being perpendicular to the Sp-orbitals form two Àp-p bonds between the two carbon atoms. Hence this results in the formation of a triple bond between the carbon atoms. Each carbon also forms a ÃÆ’sp-s bond with the hydrogen atom.
Result: Acetylene molecule has bond angle as 180 o and is linear in shape.
sp2 HYBRIDIZATION
We now discuss some examples of sp2 hybridization
1)   Boron trichloride (BCl3)
Ground state configuration being 1s2 2s2 2p1, it has only one unpaired electron. Since three unassociated electrons are required so there is promotion of one of 2s electron into the 2p sublevel by absorbing energy.
Thus the configuration becomes 1s2 2s2 2px12py1.
However to account for the trigonal planar shape of this BCl3 molecule, sp2 hybridization before bond formation was advocated.
Boron experiences sp2 hybridization by using a 2s and two 2p orbitals in the excitation state to give three half-filled sp2 hybrid orbitals which are oriented in trigonal planar symmetry. It then forms three ÃÆ’sp-p bonds with three chlorine atoms by using its half-filled sp2 hybrid orbitals. For the ÃÆ’-bond formation, the half-filled p-orbital are used.
Result: with bond angle 120o , the shape is trigonal.
2)   Ethylene (C2H4)
In the state of excitation, each carbon atom experiences sp2 hybridization by mixing 2s and two 2p orbitals to give three half-filled sp2 hybrid orbitals oriented in trigonal planar symmetry. Another unhybridized 2pz orbital is also present on each carbon perpendicular to the plane of sp2 hybrid orbitals in half-filled state.
The carbon atoms form a ÃÆ’sp2-sp2 bond with each other by using sp2 hybrid orbitals. Lateral overlapping between the unhybridized 2pz orbitals also leads to the formation of a Àp-p bond. Thus there is a double bond (ÃÆ’sp2-sp2 & Ã€p-p) between two carbon atoms. Each carbon atom also forms two ÃÆ’sp2-s bonds with two hydrogen atoms.
Result: Hence it is planar and has the ∠HCH & ∠HCC bond angles equal to 120o
 SP3 HYBRIDIZATION
1) Methane (CH4)
In the state of excitation, the carbon atom undergoes sp3 hybridization which involves the mixing of one ‘2s’ and three 2p orbitals to furnish four half-filled sp3 hybrid orbitals, which are oriented in tetrahedral symmetry in space around the carbon atom. Each one of these sp3 hybrid orbitals forms a ÃÆ’sp3-s bond with one hydrogen atom. Thus carbon forms four ÃÆ’sp3-s bonds with four hydrogen atoms. Result: The shape is tetrahedral and it has bond angle of 109o28’.
 2) Ethane (C2H6)
The case is parallel to that of methane. It experiences sp3 hybridization in the excited state which yields four sp3 hybrid orbitals in tetrahedral geometry.
The overlapping of sp3 hybrid orbitals along the inter-nuclear axis leads to the formation of a ÃÆ’sp3-sp3bond. Each carbon atom also forms three ÃÆ’sp3-s bonds with hydrogen atoms.
Result: Hence ethane has tetrahedral symmetry around each carbon with ∠HCH & ∠HCC bond angles equal to 109o28′.
3) Ammonia (NH3)
The configuration in ground state being 1s2 2s2 2px12py12pz1 shows that there are three unassociated electrons in the 2p sublevel and hence the nitrogen atom can form three bonds with three hydrogen atoms. This results in the bond angle of 90o differing from the generally reported angle of 107o48′.
Hence it was advocated for the Nitrogen to undergo sp3 hybridization of a 2s and three 2p orbitals to give four sp3 orbitals, which henceforth are organized in a tetrahedral symmetry. Since it reduces the number of repulsions so the molecule is bound to become more stable. It includes one full filled and three half filled.
Nitrogen atom forms 3 ÃÆ’sp3-s bonds with three hydrogen atoms by using three half-filled sp3 hybrid orbitals. A lone pair also exists on the nitrogen atom which requires more space than the bond pairs and belongs to full filled sp3 hybrid orbital.
Nevertheless, ∠HNH bond angle does not equal the normal tetrahedral angle: 109o28′ which is also different from the reported angle of 107o48′. The reduction in the bond angle is caused by the repulsion of lone pair over the bond pairs.
Result: The ammonia is trigonal pyramidal and has a lone pair on nitrogen atom.
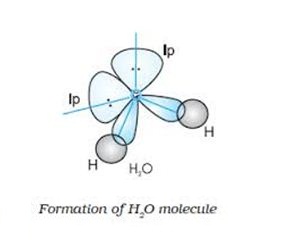
4) Water molecule (H2O)
Oxygen atom has two unpaired electrons which are free to combine with the hydrogen atoms. But the only restriction is that the resulting bond angle should be 90o. The electronic configuration of oxygen is 1s2 2s2 2px22py12pz1. The reported bond angles were 104o28′. To account this, sp3 hybridization before the bond formation was proposed.
Formation of water molecule involves the sp3 hybridization of the oxygen atom by mixing of a 2s and three 2p orbitals to furnish four sp3 hybrid orbitals oriented in tetrahedral geometry. This comprises two half-filled and two completely filled. The half-filled hybrid orbitals are then used by the oxygen atoms to form two ÃÆ’sp3-s bonds. The reported bond angle is 104o28′ instead of regular tetrahedral angle: 109o28′. It is again due to repulsions caused by two lone pairs on the bond pairs.
Result: Water molecule possesses angular shape (V shape).
Sp3d HYBRIDIZATION
1) Phosphorus pentachloride(PCl5)
The ground state electronic configuration of phosphorus atom is: 1s2 2s22p6 3s23px13py13pz1. Five unassociated electrons will suffice for the formation of PCl5 molecule. Hence the phosphorus atom undergoes excitation to promote one electron from 3s orbital to one of empty 3d orbital. As a result, in the state of excitation, the electronic configuration of ‘P’ becomes 1s2 2s22p6 3s23px13py13pz1 3d1.
In the state of excitation, the formation of five half-filled sp3d hybrid orbitals involves the intermixing of one 3s, three 3p and one 3d orbitals. They are then organized in a trigonal bipyramidal symmetry. While three orbitals are organized in trigonal planar symmetry, the rest two are kept perpendicularly above and below this plane. These half-filled sp3d orbitals aid phosphorous in forming five ÃÆ’sp3d-p bonds with chlorine atoms. Each chlorine atom makes use of half-filled 3pz orbital for the bond formation.
Result: PCl5 molecule has a trigonal bi pyramidal shape with 120o and 90o of ∠Cl – P – Cl bond angles.
SP3d2 HYBRIDIZATION
1) Sulfur hexaflouride (SF6)
The ground state configuration is 1s2 2s22p6 3s23px23py13pz1. It must have 6 unpaired electrons as the sulphur atom forms six bonds. But, the ground state of sulphur has just two unpaired electrons and hence those two only are promoted into two of the 3d orbitals (one from 3s and one from 3px). Thus the electronic configuration of ‘S’ in its 2nd excited state is 1s2 2s22p6 3s13px13py13pz13d2.
In the next state of excitation, sulfur under goes sp3d2 hybridization by mixing a 3s, three 3p and two 3d orbitals. The half-filled sp3d2 hybrid orbitals which are six in number are arranged in octahedral symmetry. Sulphur atom forms six ÃÆ’sp3d2-p bonds with 6 fluorine atoms by using these sp3d2 orbitals. The half-filled 2pz orbitals are utilized by each fluorine atom for the bond formation.
Result: SF6 is octahedral in shape with bond angles equal to 90o.
sp3d3 HYBRIDIZATION
1) Iodine heptafluoride (IF7):
The ground state electronic configuration of the Iodine atom is [Kr]4d105s25p5. Since 7 unpaired electrons are vital for the formation of IF7 , the iodine atom promotes three of its electrons (one from 5s orbital and two from 5p sublevel) into empty 5d orbitals.
This is called as the third excited state. The electronic configuration of Iodine in the third excited state can be written as: [Kr]4d105s15p35d3. In this state, the sp3d3 hybridization of iodine atom gives 7 half-filled sp3d3 hybrid orbitals in pentagonal bi-pyramidal symmetry. These will form 7 ÃÆ’sp3d3-p bonds with fluorine atoms.
Result: IF7 is pentagonal bipyramidal. The ∠F-I-F bond angles in the pentagonal plane are equal to 72o, while the two fluorine lie perpendicularly to the pentagonal plane above and below.
This post was written by Aditya Singhal, managing director of askIITians.
- Excited
- Fascinated
- Amused
- Bored
- Sad
- Angry
