One ltre of CO2 are passed over hot coke.The volume becomes 1.4litre.The composition of productis.
One ltre of CO2 are passed over hot coke.
The volume becomes 1.4litre.
The composition of productis.
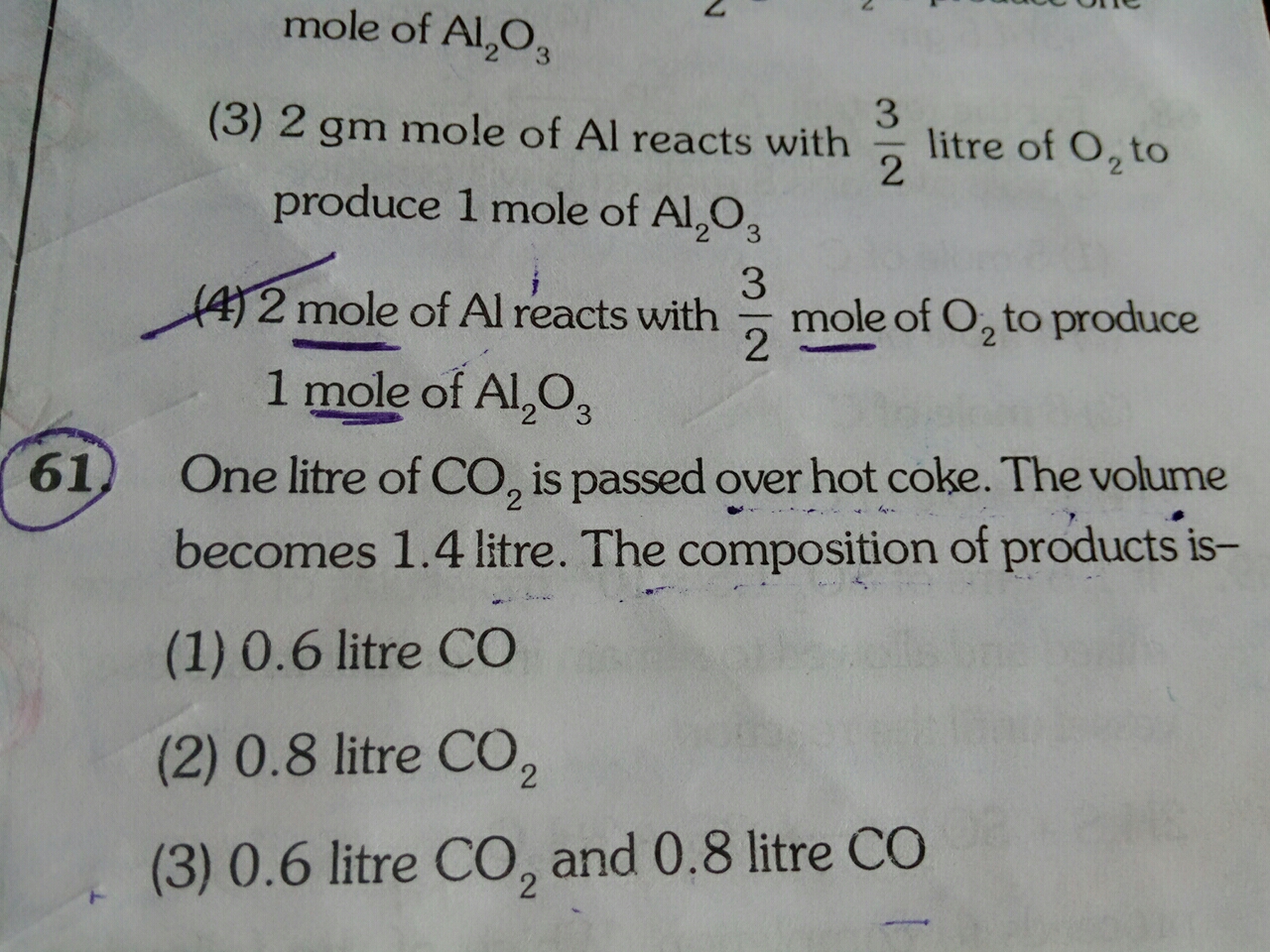

Last Activity: 3 Years ago

Last Activity: 3 Years ago


Last Activity: 3 Years ago
