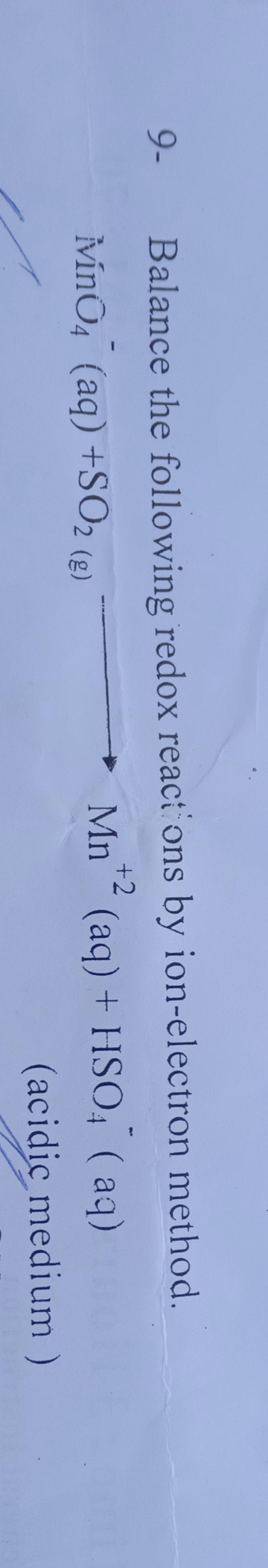Reduction half -
MnO4- --> Mn2+
MnO4- --> Mn2+ + 4H2O
MnO4- + 8H+ --> Mn2+ + 4H2O
MnO4- + 8H+ + 5e --> Mn2+ + 4H2O
2MnO4- + 16H+ + 10e --> 2Mn2+ + 8H2O
Oxidation half -
SO2 --> HSO4-
SO2 + 2H2O --> HSO4-
SO2 + 2H2O --> HSO4- + 3H+
SO2 + 2H2O --> HSO4- + 3H+ + 2e
5SO2 + 10H2O --> 5HSO4- + 15H+ + 10e
Adding both eq ,
2MnO4- + 16H+ + 10e + 5SO2 + 10H2O -----> 2Mn2+ + 8H2O + 5HSO4- + 15H+ + 10e
2MnO4- + H+ + 5SO2 + 2H2O -----> 2Mn2+ + 5HSO4-