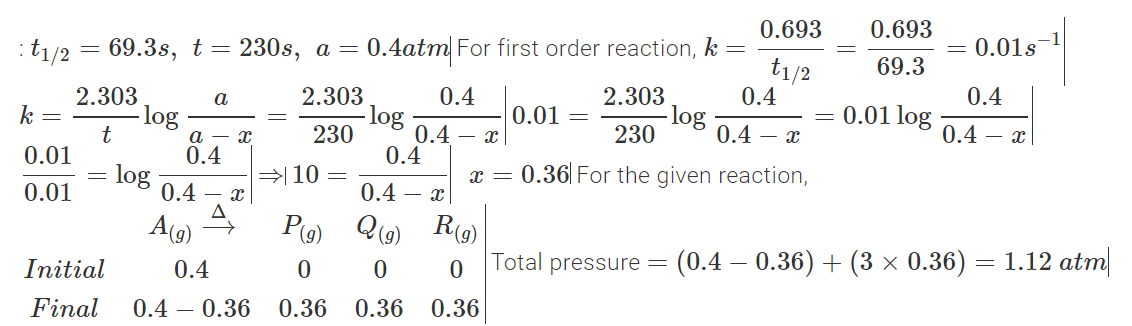A → P+Q+R follows first order kinetics with a half life of 69.3 seconds at 500 C. STarting from the gas A enclosed in a container at 500 C and at a pressure of 0.4 atm the total presurre of the system after 230 seconds will be
A → P+Q+R follows first order kinetics with a half life of 69.3 seconds at 500 C. STarting from the gas A enclosed in a container at 500 C and at a pressure of 0.4 atm the total presurre of the system after 230 seconds will be