Dear student,
The basic hydrogen energy level structure is in agreement with the Bohr model. Common pictures are those of a shell structure with each main shell associated with a value of the principal quantum number n.
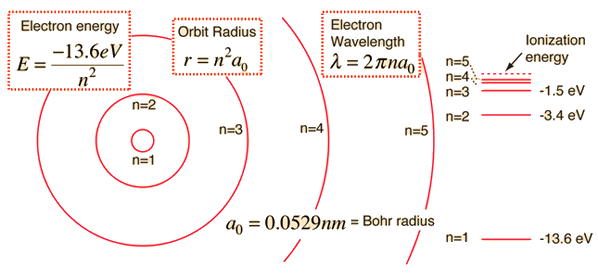
This Bohr model picture of the orbits has some usefulness for visualization so long as it is realized that the "orbits" and the "orbit radius" just represent the most probable values of a considerable range of values. If the radial probabilities for the states are used to make sure you understand the distributions of the probability, then the Bohr picture can be superimposed on that as a kind of conceptual skeleton.




