Dear Student

where, p is the pressure,  is the molar volume of the gas, T is the temperature, and R is the gas constant.
is the molar volume of the gas, T is the temperature, and R is the gas constant.
For an ideal gas the compressibility factor is Z = 1 per definition
The reduced temperature and pressure are defined as:
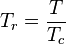 and
and 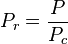
Tc and Pc are known as the critical temperature and critical pressure of a gas. They are characteristics of each specific gas with Tc being the temperature above which it is not possible to liquify a given gas and Pc is the minimum pressure required to liquify a given gas at its critical temperature.
 and
and 
now you can eliminate T and P and put in terms of critical temp and pressure.
All the best.
AKASH GOYAL
AskiitiansExpert-IIT Delhi
Please feel free to post as many doubts on our discussion forum as you can. We are all IITians and here to help you in your IIT JEE preparation.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively :Click here to download the toolbar..




