Other Related Questions on physical chemistry

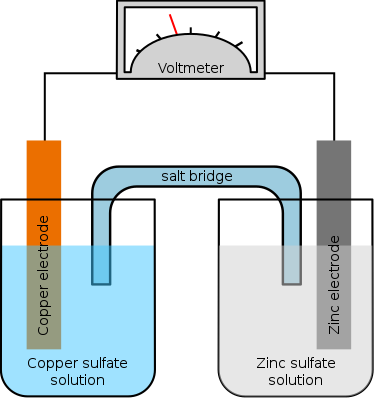
Which of the following aqueous solutions remains neutral after
electrolysis
Which of the following aqueous solutions remains neutral after
electrolysis
electrolysis
physical chemistry
0 Answer Available
Last Activity: 3 Years ago

In the reaction A + 2B ⇌ 2C, if 2 moles of A, 3 moles of B and 2 moles of C are placed in a 2 L flask and
the equilibrium concentration of C is 0.5 mol/L. The equilibrium constant (KC) for the reaction is.....and please tell how you know that the reaction is going forward .. imean how to calculate the reaction quotient ???
In the reaction A + 2B ⇌ 2C, if 2 moles of A, 3 moles of B and 2 moles of C are placed in a 2 L flask and
the equilibrium concentration of C is 0.5 mol/L. The equilibrium constant (KC) for the reaction is.....and please tell how you know that the reaction is going forward .. imean how to calculate the reaction quotient ???
the equilibrium concentration of C is 0.5 mol/L. The equilibrium constant (KC) for the reaction is.....and please tell how you know that the reaction is going forward .. imean how to calculate the reaction quotient ???
physical chemistry
1 Answer Available
Last Activity: 3 Years ago


18 19 gram mixture of na2co3 and nahco3 on heating gives 2.2 gram CO2 gas find the percent nahco3 (by mass) in mixture
18 19 gram mixture of na2co3 and nahco3 on heating gives 2.2 gram CO2 gas find the percent nahco3 (by mass) in mixture
physical chemistry
1 Answer Available
Last Activity: 3 Years ago