Dear anuj,
For unit concentration of reactants rate of the reaction is equal to rate constant or specific reaction rate.
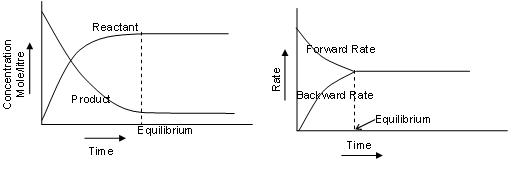
At equilibrium the rate of both forward and backward reactions become equal and after achieving equilibria, the concentration of reactants and products remains constant as shown in figure.
Active mass is the molar concentration of the reacting substances actually participating in the reaction.
Hence
Active mass = number of moles/volume in litres
and Active mass of solid is taken as unity.
We are all IITians and here to help you in your IIT JEE preparation.
All the best.
If you like this answer please approve it....
win exciting gifts by answering the questions on Discussion Forum
Sagar Singh
B.Tech IIT Delhi




