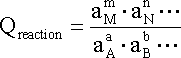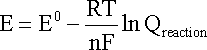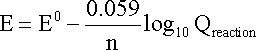ONE half cell in a voltaic cell is constructed from a silver wire dipped in a silver nitrate solution of unknown concentration. its other half cell consists of a zinc electrode dipped in 1.0 M solutionof zinc nitrate. a voltage of 1.48 is measured for this cell. calculate concentration of silver nitrate. {standard electrode potentials are given: Zn2+\Zn= -0.76 V . Ag+\Ag = 0.80 V.}
ONE half cell in a voltaic cell is constructed from a silver wire dipped in a silver nitrate solution of unknown concentration. its other half cell consists of a zinc electrode dipped in 1.0 M solutionof zinc nitrate. a voltage of 1.48 is measured for this cell. calculate concentration of silver nitrate. {standard electrode potentials are given: Zn2+\Zn= -0.76 V . Ag+\Ag = 0.80 V.}