Solved Examples on Redox Reactions and Electrochemistry
Question:1) Given

Based on the data given above, strongest oxidising agent will be. (IIT JEE-2013)
1) Cr3+
2) Mn2+
3) MnO4-
4) Cl-
Answer: (3)
Solution:
More positive value of standard reduction potential indicated the poor tendency of the species to
undergo reduction. In the given data, MnO4- has the highest positive value of standard
reduction potential this means that it has least tendency to undergo reduction and thus highest
tendency to undergo oxidation among the given species, thus it is the strongest oxidising agent.
Hence, the correct option is 3.
Question: 2) The reaction of white phosphorous with aqueous NaOH gives phosphine along with another phosphorous containing compound. The reaction type; the oxidation on states of phosphorus in phosphine and the other product are respectively (IIT JEE -2012)
(A) redox reaction; −3 and −5
(B) redox reaction; +3 and +5 .
(C) disproportionation reaction; −3 and +5.
(D) disproportionation reaction; −3 and +3 .
Answer: C
Solution: The Balanced Reaction:

It is clear from the balanced reaction that it is a disproportionation reaction as P undergo both oxidation as well as reduction in this reaction.
NaH2PO2 formed in this reaction is less stable and decompose to from two products Na2HPO4 and PH3. Oxidation number of P in Na2HPO4 is +5.
Hence, the correct option is C.
Question: 3) The solubility product (Ksp; moldm–9) of MX2at 298 K based on the information available for the given concentration cell is (take 2.303 ×R ×298/F = 0.059 V). (IIT JEE -2012)
1) 1 ×10–15
2) 4 ×10–15
3) 1 ×10–12
4) 4 ×10–12
Answer: C
Solution:
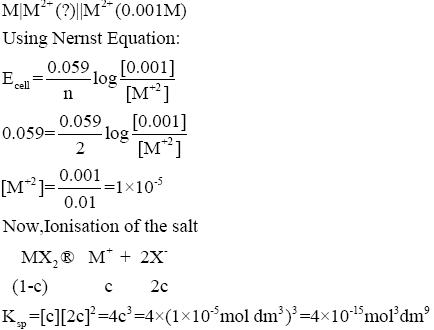
Hence, the correct option is C.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

