Application of bond energies
(i) Determination of enthalpies of reactions
Suppose we want to determine the enthalpy of the reaction.
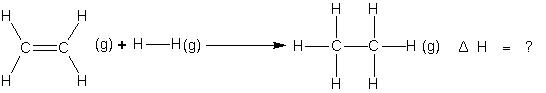
If bond energies given for C ¾ C, C = C, C¾H, and H ¾ H are 347.3, 615.0, 416.2 and 435.1KJ mol-1 respectively.
ΔH = ΔHC=C + ΔHH–H + 4ΔHC–H – (ΔHC–C + 6ΔHC–H)
= (615.0 + 435.1) – (347.3 + 832.4) => –129.6 KJ
(ii) Determination of enthalpies of formation of compounds
Consider the formation of acetone.
H O H
| || |
| |
ΔHf = [3ΔHH–H + 1/2 ΔH0–0 + 3ΔHC(s)—>C(g)] – [2ΔHC–C + 6ΔHC–H + ΔHC=O]
by putting the value of different bond energies you can determine the ΔHf .
(iii) Determination of resonance energy
If a compound exhibits resonance, there is a considerable difference between the enthalpies of formation as calculated from bond energies and those determined experimentally. As an example we may consider the dissociation of benzene.
C6H6 (g) ———> 6C(g) + 6H(g)
Assuming that benzene ring consists of three single and three double bonds (Kekule’s structure) the calculated dissociation energy comes out to be 5384.1 KJ from bond energies data.
ΔHd = 3ΔHC–C + 3ΔHC=C + 6ΔHC–H
The experimental value is known to be 5535.1 KJ/mol. Evidently, the energy required for the dissociation of benzene is 151 KJ more that the calculated value. The difference of 151 KJ gives the resonance energy of benzene.
Exercise:
Calculate the enthapy of combustion of benzene (l) on the basis of the following.
(i) Resonance energy of benzene (l) = – 152 kJ mole–1
(ii) Enthalpy of hydrogenation of cyclohexene (l) = – 119 kJ mole–1
(iii) (ΔHf0)C6H12 = – 156 kJ mole–1
(iv) (ΔH0f)H2O = – 285.8 kJ mole–1
(v) (ΔHf0)CO2 = – 393.5 kJ mole–1
To read more, Buy study materials of Thermodynamics comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More
