Classification of Solids
Table of Content |
-
 Solids are broadly classified into two types crystalline solids and amorphous solids.
Solids are broadly classified into two types crystalline solids and amorphous solids.
-
A crystalline solid is a substance whose constituent particles possess regular orderly arrangement e.g. Sodium chloride, sucrose, diamond etc.
-
An amorphous solid is a substance whose constituent particles do not possess a regular orderly arrangement e.g. glass, plastics, rubber, starch, and proteins.
-
Though amorphous solids do not possess long range regularity, in some cases they may possess small regions of orderly arrangement. These crystalline parts of an otherwise amorphous solid are known as crystallites.
-
An amorphous solid does not possess a sharp melting point. It undergoes liquefication over a broad range of temperature.
-
The amorphous solid do not possess any characteristic heat of fusion.
-
When an amorphous solid is cut with the help of sharp edged knife it results in an irregular cut.
-
Amorphous substances are also, sometimes, referred to as super cooled liquids because they posses disorderly arrangement like liquids. In fact many amorphous solids such as glass are capable flowing. Careful examination of the window panes of very old houses reveals that the panes are thicker at the bottom than at the top because the glass has flown under constant influence of gravity.
Distinction between Crystalline and Amorphous Solids
Uses of Amorphous Solids
-
Amorphous solids such as glass and plastics are very important materials and are widely used in construction, house ware, laboratory ware etc.
-
Amorphous silica is likely to be the best material for converting sunlight into electricity (photovoltaic).
-
Another well known amorphous solid is rubber which is used in making tyres shoes soles etc.
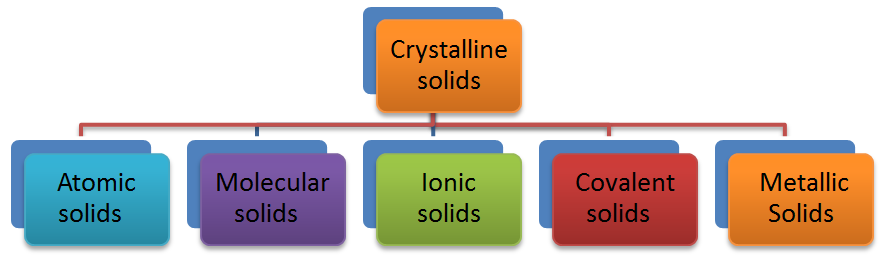
Classification of Crystalline Solids based on Different Binding Forces
Crystalline solids can be classified into different categories depending upon the type of constituent particles and the nature of attractive forces operating between them.
Atomic Solids
In these solids the constituent particles are atoms. These closely packed atoms are held up by London dispersion forces. Some examples are crystals of noble gases. Such solids are very soft, possess very low melting points and poor conductors of heat and electricity.
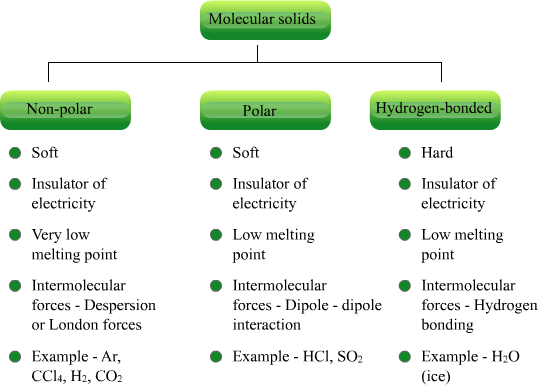
Molecular Solids
In these solids, the constituent particles which pack up together are molecules of the substance. These molecules may be non – polar (dipole moment = 0) such as etc. or they may be polar (dipole moment > 0) like etc.
In case of non – polar molecules, the attractive forces operating between the molecules are Vander Waal forces (also called dispersion forces). The example of such solids are : dry ice (Solid, iodine (crystals).
In case of polar molecules, the attractive forces operating between the molecules in solid state are dipole – dipole forces. The examples of such solids are : solid, solid HCl. However, in some solids with polar molecules, the interparticle forces are hydrogen bonds. The examples of such solids are ice; solid hydrogen fluoride (HF); solid ammonia, etc.
Characteristics of Molecular Solids
-
Some of the general characteristics of molecular solids are :
-
Their melting points are low to moderately high. The melting points of solids with non – polar molecules are relatively low whereas solids with polar molecules have moderately high melting points.
-
They are generally bad conductors of heat and electricity.
-
They have generally low density.
Ionic Solids
 In ionic solids, the constituent particles are ions of opposite charges. Each ion is surrounded by a definite number of ions of opposite charge.
In ionic solids, the constituent particles are ions of opposite charges. Each ion is surrounded by a definite number of ions of opposite charge.
The number of ions that surround a particular ion of opposite charge its called co – ordination number of the ion. For example, in sodium chloride crystal each sodium ion is surrounded by six chloride ions. Hence coordination number of is 6. At the same time each chloride ion is surrounded by six ions. Therefore the co – ordination number of ion is also 6. However, in calcium fluoride crystal each ion is surrounded by eight fluoride ions and each ion is surrounded by four ions.
Thus, in crystal co – ordination numbers of and ions are respectively 4 and 8. The interparticle forces in ionic solids are ionic bonds operating between the ions of opposite charges some examples of ionic solids are : sodium chloride (NaCl) ; ceasium chloride (CsCl), zinc sulphide (ZnS), calcium fluoride, etc.
Characteristics of Ionic Solids
-
Some common characteristics of ionic solids are as follows:
-
They have high melting points.
-
They are poor conductors of electricity in solid state, however they become good conductors of electricity in molten state or in dissolved state.
-
They are generally soluble in polar solvents like water.
Covalent Solids
 In these types of solids the constituent particles are atoms of same or different elements connected to each other by covalent bond network.
In these types of solids the constituent particles are atoms of same or different elements connected to each other by covalent bond network.
For example, in diamond only carbon atoms constitute the covalent network while carborundum covalent bond network is constituted by silicon and carbon atoms. Obviously, the interparticle forces operating in these solids are covalent bonds.
These solids are also called network solids because the covalent bonds extend in three dimensions forming a giant interlocking structure. Some examples of covalent solids are :
Diamond, silicon carbide, aluminium nitrite etc.
Characteristics of Covalent Solids
Some common characteristics of covalent solids are :
-
They are very hard. Diamond is the hardest naturally occurring substance.
-
They have very high melting points.
-
They are poor conductors of heat and electricity.
-
They have high heats of fusion.
Metallic Solids
In these type of solids, the constituent particles are metal atoms. The interparticle forces in these solids are metallic bonds. In the metallic crystals the metal atoms occupy the fixed positions but their valence electrons are mobile.
The close packed assembly of metal kernels (part of metal atom without valence electrons) remain immersed in the sea of mobile valence electrons. The attractive force between the kernels and mobile valence electrons is termed as metallic bond.
Characteristics of Metallic Solids
-
The common characteristics of metallic solids are as follows:
-
They generally range from soft to very hard.
-
They are malleable and ductile.
-
They are good conductors of heat and electricity.
-
They possess bright lustre.
-
They have high melting and boiling points.
-
They have moderate heats of fusion.
-
The summary of classification of solids on the basis of interparticle forces is given in
Classification of Solids on the Basis of Binding Forces
|
CrystalClassification |
Unit Particles |
Binding Forces |
Properties |
Examples |
|
Atomic |
Atoms |
London dispersion forces |
Soft, very low melting, poor thermal and electrical conductors |
Noble gases |
|
Molecular |
Polar or |
Vander Waal’s forces (London dispersion, dipole – dipole forces hydrogen bonds) |
Fairly soft, low to moderately high melting points, poor thermal and electrical conductors |
Dry ice (solid, methane |
|
Ionic |
Positive and negative ions |
Ionic bonds |
Hard and brittle, high melting points, high heats of fusion, poor thermal and electrical conductors |
NaCl, ZnS |
|
Covalent |
Atoms that are connected in covalent bond network |
Covalent bonds |
Very hard, very high melting points, poor thermal and electrical conductors |
Diamond, quartz, silicon |
|
Metallic |
Cations in electron cloud |
Metallic bonds |
Soft to very hard, low to very high melting points, excellent thermal and electrical conductors, malleable and ductile |
All metallic elements, for example, Cu, Fe, Zn |

Question 1: Amorphous solids..
a. have sharp melting point.
b. undergo clean cleavage.
c. have regular geometry.
d. are isotropic in nature.
Question 2: Which of the following solids is not crystalline?
a. copper
b. soldium chloride
c. diamond
d. silica
Question 3: Which of the following statements regarding covalent solids is incorrect?
a. They are very hard.
b. They have very high melting points.
c. They are good conductors of heat
d. They have high heats of fusion.
Question 4: Dry ice is a
a. molecular solid
b. ionic solid
c. atomic solid
d. metallic solids
Question 5: quartz is a
a. ionic solid
b. covalent solid
c. atomic solid

| Q.1 | Q.2 | Q.3 | Q.4 | Q.5 |
| d | d | c | a | b |
Related Resources
-
Click here to get Syllabus of IIT JEE
-
Have a look at Books of Chemistry for IIT JEE
To read more, Buy study materials of Solid State comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.