The s-Block Elements
Table of Content |
|
|
Group 1 Elements: Alkali Metals
Group 1 elements are known as Alkali Metals. It includes Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs) and Francium (Fr). This group lies in the s block of the periodic table.
Fig. 1. Periodic table
-
They are shiny, highly reactive metals.
-
They are kept in certain solutions such as oil to prevent reactivity with the air.
-
They are soft and can be cut via knife.
-
Sodium is abundant and francium is rare.
Physical Properties of Alkali Metals:
-
They have metallic bonding due to which they are conducting in nature.
-
They produce different colors with flame test.
-
Electronegativity and ionization enthalpy both decreases from lithium to francium as size increases.
-
Nuclear charge also decreases as one moves from lithium to francium due to increase in the size of the atom.
-
After losing one valence electron, they can attain noble gas configuration.
Chemical Properties of Alkali Metals:
-
Alkali metals reacts with oxygen to form oxides, peroxides and superoxides. Lithium only forms monoxides. But other alkali metals can form peroxides and superoxides.
4 Li +O2 → 2Li2 O (oxide)
-
They form hydroxides with water.
2Li +2H2O → 2LiOH+H2
-
They form hydrides with hydrogen.
2 Li + H2 → 2 LiH
-
Due to their low first ionization energy, they can react vigorously with halogen to form halides.
2Na(s) + Cl2(g) → 2NaCl(s)
-
Alkali metals dissolve in liquid ammonia to form ammoniated ions which impart blue color to the solution.
M + ( x + y ) NH3 → M+ ( NH3 )x + e-(NH3)y
-
Alkali metals also forms salts with oxoacids.
Uses of Alkali Metals:
-
They are used to make alloys.
-
Radium is used to treat cancer cells.
-
Potassium helps in opening and closing of stomata.
-
Potassium hydroxide works as a precipitating agent.
General Characteristics of Compounds of Alkali Metals
-
All monoxides of alkali metals are basic in nature.
-
Hydroxides of alkali metals behave like a strong base.
-
They are miscible in polar solvents.
-
They are electropositive in nature and metallic character increases from lithium to francium.
Anomalous Properties of Lithium
Lithium show diagonal relationship with magnesium. There are many reasons for this relationship, which are as follows:
-
Lithium and magnesium has comparable boiling points.
-
They both are equally electropositive.
-
They both forms monoxides when exposed in air.
2Mg + O2 → 2MgO
4 Li +O2 → 2Li2 O
-
They both forms nitrides with nitrogen known as Lithium Nitride and Magnesium Nitride.
6 Li + N2 → 2 Li3N
-
Lithium and magnesium both do not form superoxides.
-
Lithium chloride (LiCl) and magnesium chloride (MgCl2) both are soluble in ethanol.
Difference between Lithium and Other Alkali Metals:
-
Lithium is harder comparable to other alkali metals.
-
Lithium is least reactive out of all alkali metals.
-
It is a strong reducing agent as compared to other alkali metals.
-
It is the only alkali metal that form the monoxide, Li2O.
4Li(s) + O2(g) → Li2O(s)
-
It forms, lithium nitride, Li3N. But other alkali metals do not form nitrides
-
It does not form solid hydrogen carbonates compared to other alkali metals.
-
Lithium does not form ethynide from ethyne as compared to other alkali metals.
4 LiNO3 → 2 Li2O + 4NO2 + O2
-
Lithium reacts slowly with bromine but other alkali metals do not react.
Some Important Compounds of Sodium
The important compounds of sodium are as follows:
-
Sodium Carbonate
-
Sodium Chloride
-
Sodium Hydroxide
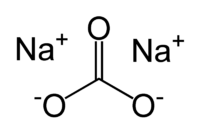
Sodium Carbonate (Na2CO3.10H2O)
- Commonly known as Washing Soda.
Fig. 2. Structure of Sodium Carbonate
- It is synthesized by Solvay process. During this process, sodium carbonate is synthesized using sodium chloride and calcium carbonate as a precursor.
2 NaCl + CaCO3→ Na2CO3+ CaCl2
The steps for the formation of Na2CO3 are as follows:
-
During first step, sodium chloride reacts with ammonia, carbon-dioxide and water to form sodium bi-carbonate.
NaCI + CO2 + NH3 + H2O → NaHCO3 + NH4Cl
-
During the second step, calcium carbonate is converted into calcium oxide and calcium carbonate.
CaCO3 → CO2 + CaO
-
Sodium bicarbonate reacts with calcium oxide from the step 2 to form ammonia, calcium chloride and water
2 NH4Cl + CaO → 2 NH3 + CaCl2 + H2O
-
Sodium bicarbonate finally decompose into sodium carbonate.
2 NaHCO3 → Na2CO3 + H2O + CO2
-
Sodium carbonate is soluble in water.
Uses of Sodium Carbonate:
-
Used in manufacturing of glass.
-
Synthesis of borax, soap, and caustic soda also uses sodium carbonate as one of the ingredients.
-
Sodium carbonate is also used in paint and textiles industry.
Sodium Chloride (NaCl)
-
Sodium chloride better known as Common Salt.
-
Reverse osmosis is one of the method other than evaporation of sea water to obtain salt.
Fig. 3. Structure of crystal of Sodium Chloride
Uses of Sodium Chloride:
-
It is used as common salt for domestic purpose.
-
It is used for the preparation of Na2O2, NaOH and Na2CO3.
Sodium Hydroxide (NaOH)
-
Commonly known Caustic Soda.
-
Castner-Kellner Cell is an electrolysis method to synthesize sodium hydroxide.
Fig. 4. Castner-Kellner Cell
-
Sodium hydroxide is a white solid which is soluble in water.
-
Sodium hydroxide reacts with carbon-dioxide to form Na2CO3.
NaOH + CO2 → Na2CO3
Uses of Sodium Hydroxide:
-
Sodium industries used sodium hydroxide.
-
Used in petroleum refining.
-
Used in textiles industries such as cotton industries.
-
Used as a precipitating agent in the laboratories.
-
Sodium hydroxide is used during preparation of fats and oils.
Sodium Hydrogen Carbonate(NaHCO3):
2 NaHCO3(s) → CO2(g) + H2O(g) + Na2CO3(s)
-
Used as an antiseptic.
-
Used as a fire extinguisher.
-
Used in bakeries to prepare pastries, cake etc.
Group 2 Elements: Alkaline Earth Metals
Group 2 elements are known as Alkaline Earth Metals. It includes beryllium, magnesium, calcium, strontium, barium, and radium. The oxidation state of alkaline earth metals is +2. Their outer electronic configuration is ns2.
Physical Properties of Alkaline Earth Metals:
-
Alkaline earth metals are silvery, white in color.
-
There melting and boiling point is higher compared to alkali metals.
-
They are electropositive in nature.
-
They have metallic bonding which make them conductive.
-
They give different color with flame test. Calcium gives brick red color, strontium gives crimson color and barium gives apple green color.
Chemical Properties of Alkaline Earth Metals:
- Beryllium and magnesium do not react with oxygen.
2Ca(s) + O2(g) → 2CaO(s)
- Alkaline earth metals react with halogen to form halides.
Be(s) + Cl2(g) → BeCl2(s)
-
Like alkali metals, alkaline earth metals react with hydrogen to form halides. But beryllium do not react with hydrogen.
-
They are strong reducing agents.
-
They form blue black color in ammonia, due to the formation of the ammoniated ions.
Uses of Alkaline Earth Metals:
-
Calcium is important for bones, teeth, and muscle contraction.
-
Milk of magnesia is used as antacid.
-
Magnesium carbonate is a component of toothpaste.
-
Strontium is used in glass wares.
Anomalous Behavior of Beryllium
Beryllium shows diagonal relation with aluminum.
Difference between Beryllium and other Alkaline Earth Metals:
-
Beryllium is the lightest of all group 2 elements.
-
It has higher melting and boiling points in comparison to other elements in group 2.
-
BeO is amphoteric whereas oxides of other alkaline earth metals are strong alkali.
-
Beryllium do not impart color during flame test.
-
Beryllium is small in size with high ionization enthalpy.
-
Beryllium do not liberate hydrogen from acids
Similarities between Beryllium and Magnesium/Diagonal Relationship of Beryllium with Aluminum:
-
Beryllium and aluminum both reacts with nitric acid
-
Both beryllium and aluminum reacts with an alkali to form beryllate and aluminate.
Be3N2 + 6 NaOH →3 Na2BeO2 + 2 NH3
2 Al + 2 NaOH + 2 H2O → 2 NaAlO2 + 3 H2
- Both beryllium and aluminum combines with halogens to form polymeric halides.
Be(s) + Cl2(g) → BeCl2(s)
2Al(s) + 3Br2(l) → Al2Br6
Fig. 5. Polymeric Structure of Beryllium Chloride
Fig. 6. Polymeric Structure of Aluminum Bromide
-
Beryllium and aluminum both has strong tendency to form complexes.
-
Both aluminum and beryllium form nitrides by liberating ammonia in presence of water
Be3N2 + 6 H2O → 3 Be(OH)2 + 2 NH3
AlN + 3H2O → Al(OH)3+ NH3
-
Carbides of beryllium and aluminum both gives methane in presence of water.
Al4C3 + 12 H2O → 4 Al(OH)3 + 3 CH4
Biological Importance of Sodium and Potassium
-
Sodium ions are primary found outside the human cells.
-
Sodium maintains the electrolyte balance in the body.
-
Sodium chloride is used as a preservative in pickling.
-
A drop-in sodium levels in the blood plasma below a reference value is known as hyponatremia. Hyponatremia leads to headache, nausea, seizures etc.
- Potassium ions are primarily found inside the cell.
-
Potassium ions maintain the osmolarity.
-
They also regulate the opening and the closing of the stomata.
-
Potassium ions acts as cofactor for enzymes of glycolysis.
-
Potassium is important in skeleton and muscle contraction.
-
Diets with low potassium leads to hypertension.
Biological Importance of Magnesium and Calcium
-
Magnesium is essential for the activity of enzymes.
-
It is the central atom present in chlorophyll.
-
It is essential for the synthesis of ATP
-
Responsible for the stability of DNA.
-
Maintains the electrolyte balance in the body.
-
Magnesium deficiency is associated with insomnia.
-
Deficiency also leads to abnormal heart beats.
Uses of Magnesium:
-
Magnesium alloys are used in making flares, fuse for thermite.
-
Preparation of malleable cast iron.
-
Used to remove Sulphur.
-
As a reducing agent to separate uranium.
-
Needed for blood glucose control.
Biological Importance of Calcium:
-
Component of cell wall.
-
Required for blood clotting.
-
Helps in muscle contraction.
-
Helps in proper heart and nerve functions.
-
Calcium is essential for growth of bones and teeth.
The ideal ratio of calcium and magnesium is 1:1. Both works antagonistic to each other. For Example, if calcium contracts muscle, magnesium relaxes muscle.
Some Important Compounds of Calcium
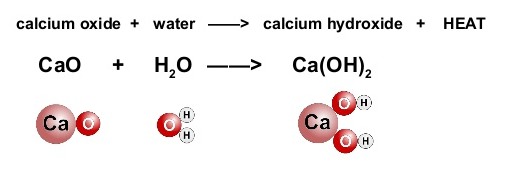
Calcium Oxide (CaO):
-
Also, known as Quick Lime.
-
Calcium carbonate on heating forms calcium oxide and carbon-dioxide.
Fig. 5. Formation of Calcium Hydroxide from Calcium Oxide
-
Calcium oxide on hydrolysis forms calcium hydroxide.
-
Calcium oxide on reaction with carbon-dioxide forms calcium carbonate.
-
Important ingredient of preparing cement.
-
Calcium Oxide is used in the manufacturing of sodium carbonate.
Calcium Hydroxide(Ca(OH2):
-
Also, known as Slaked Lime.
-
Calcium oxide on hydrolysis forms calcium hydroxide.

-
The lime water is diluted solution of calcium hydroxide.
-
Hypochlorite is one of the constituent of bleaching powder. Passing chlorine through calcium hydroxide forms hypochlorite
-
It is used to prepare building material mortar.
-
Calcium hydroxide has disinfectant property.
Calcium Carbonate(CaCO3):
-
Limestone, marble, chalk can be commonly known as Calcium Carbonate.
-
Calcium carbonate is insoluble in water.
-
Decomposition of calcium carbonate forms quick lime, that is, calcium oxide and carbon-dioxide.
-
Marbles made up of calcium carbonates is used as building material.
-
Calcium carbonate is used as an antacid.
-
It is one of the constituent of toothpaste, chewing gum etc.
Calcium Sulpahte (CaSO4):
-
It is commonly known as plaster of Paris.
-
Heating of gypsum, that is, CaSO4.2H2O forms calcium sulphate.
2 CaSO4 .2H2O → 2 CaSO4.H2O + 3H2O
-
Anhydrous calcium suphate is known as “Dead Burnt Plaster”.
-
It is used in building industries for making POPs.
-
It is also used for fixing bone parts after fracture.
-
Used in statue making.
Cement:
-
Commonly known as Portland cement.
-
Commonly used as building material.
-
The main constituents of cement are silicon dioxide, calcium oxide, aluminum, iron, and magnesium.
-
Cement is dicalcium silicate, tricalcium silicate and, tricalcalcium aluminate.
-
It is the most common material used during plastering.
-
It is used in construction of dams, bridges, and buildings.
Watch this Video for more reference
More Readings