Oxoacids of Phosphorus
Table of Content |
Introduction to Oxoacids of Phosphorus
Phosphorus forms various oxoacids. They are hypophosphorous acid (H3PO2), Phosphorous acid (H3PO3), Hypophosphoric acid (H3PO4), pyrophosphoric acid (H4P2O7) and meta phosphoric acid (HPO2) n.
Oxoacids of Phosphorus are given in table beneath.
Among the oxoacids of phosphorus, orthophosphoric acid is the most critical and is utilized as a part of the produce of phosphate manures.
Hypo Phosphorous Acid (H3PO2) or Phosphinic Acid
It is set up by the oxidation of phosphine by iodine within the sight of figured amount of water. It is a monobasic acid.
PH3 + 2I2 + 2H2O -----------> H3PO2 + 4 HI
Fig. 1: Hypo Phosphorous Acid
Phosphorous Acid (H3PO3) or Phosphonic Acid
It is set up by hydrolysis of phosphorous trioxide (P4O6). Phosphorous acid is dibasic in nature.
P4O6 + 6H2O ----------> 4H3PO3
Fig. 2: Phosphorous Acid
Hypophosphoric Acid (H4P2O6)
It is set up by controlled oxidation of red phosphorus with sodium chlorite arrangement when disodium salt of the hypophosphoric acid is obtained it is passed through cation exchanger to yield hypophosphoric acid. Hypophosphoric acid is tetrabasic in nature.
2P + 2NaClO2 + 2H2O ---------> Na2H2P2O6 + 2HCl
Na2H2P2O6 + 2H -----resin-----> H4P2O6 + 2Na – resin
Fig. 3: Hypophosphoric Acid
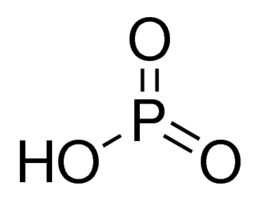
Orthophosphoric Acid (H3PO4)
It is set up by treating P4O10 with bubbled water. It is a tribasic acid.
P4O10 + 6H2O ----------> 4H3PO4
Fig. 4: Orthophosphoric Acid
Pyrophosphoric Acid (H4P2O7)
It is set up by heating orthophosphonic acid at about 250oC. It is a tetrabasic acid.
2H3PO4 ---------> H4P2O7 + H2O
Fig. 5: Pyrophosphoric Acid
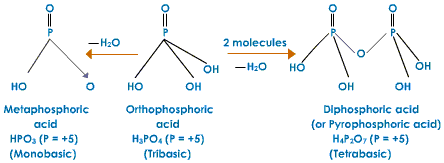
Meta Phosphoric (HPO3)n
It is formed by warming orthophosphoric acid to around 850 K. Metaphosphoric acid does not exist as monomer. It exists as cyclic trimer, cyclic tetramer or polymer.
H3PO4 ---------> HPO3 + H2O
Fig. 6: Metaphosphoric Acid
The structures of oxoacids of phosphorus are efficiently given ahead:
Fig. 7: Structure of oxoacids of phosphorus with their oxidation states and basic nature
The formulae of oxoacids of P can be remembered as:
-
Meta Acid is utilized for the acid got by the loss of one water particle.
-
Pyro Acid is utilized for the acid acquired by warming two atoms with the loss of one water particle.
-
Hypo is by and large utilized for the acid having lower oxygen content than the parent acid.
In any case, it might be noticed that metaphosphoric acid does not exist as basic monomer; it exists as cyclo metaphosphoric acid or poly metaphosphoric acid.
 |
 |
Fig. 8: Forms of Metaphosphoric Acid |
Fig. 8: Forms of Metaphosphoric Acid |
Watch this Video for more reference
More Readings