Group 17 Elements
Table of Content |
|
|
Introduction to Group 17 Elements
Group seventeen elements include fluorine, chlorine, bromine, iodine and astatine.
Fig. 1: Group 17 elements
These elements are on the whole alluded to as the "halogens" as they react with metals to give salts.
Electronic Configuration
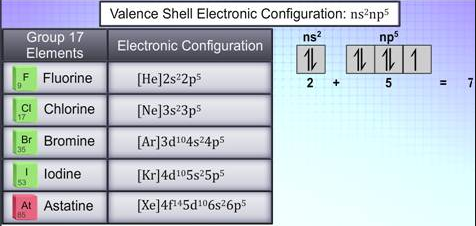
ns2 np5 is the valence shell electronic configuration of these elements which makes clear why there are seven electrons in the outer shell.
Fig. 2: Electronic configuration of group 17 elements
The valence shell is short of an octet configuration by one electron. As these elements require one electron to achieve stable octet or closest ideal gas configuration, they have a strong inclination to either increase one electron to shape an ionic bond or impart an electron to another atom to frame a covalent bond.
The halogens constitute the most reactive group of non-metals. The high reactivity of the halogens is credited to their strong inclination to pick up or share an electron to accomplish closest inert gas configuration.
Occurrence
Inferable from their high reactivity, the halogens do not exist in Free State, yet in the consolidated state in nature, aside from astatine.
Astatine is radioactive in nature.
Fluorine is the 13th and chlorine the 20th most rich element by weight in the crust of the world.
Fluorineexist broadly as insoluble fluorides, for example, cryolite(Na3AlF6), fluorspar(CaF2), and fluoroapatite (Ca5(PO4)3F). Of these, the primary source is fluorspar. Little measures of fluorine are available in soil, plants of stream water, and the bones and teeth of creatures.
Chlorine, bromine, and iodine are available in ocean water as chlorides, bromides, and iodides of profoundly dynamic metals like sodium, potassium, magnesium and calcium. Of these, the richest is sodium chloride. Ocean water comprises around 1.5 % by weight of sodium chloride.
The dry beds of oceans additionally contain vast stores of sodium chloride alongside littler extents of calcium chloride and carnallite (KCl.MgCl2.6H2O).
Iodides are found in trace amounts in ocean water. The primary wellspring of iodine is ocean weeds and crude chile salt petre.
Atomic Properties
Patterns of a portion of the atomic properties of group 17 elements:
Atomic properties incorporate ionic and atomic radii, electron gain enthalpy, ionization enthalpy, and electro-negativity.
Trend of Ionic and Atomic Radii
As we move down the group, the nuclear radii, and ionic radii increment because of the addition of another vital energy level in each progressive element.
These elements have the smallest atomic radii when contrasted with different elements in the relating periods. This is a direct result of greatest powerful atomic charge.
Fig. 3: Trends in atomic and ionic radii
Ionisation Enthalpy
These elements demonstrate high estimations of ionization enthalpy. As an outcome, the molecules of these elements tend to lose electrons and frame positive particles. As we move down the group, the value of ionization energy diminishes. This is because of the steady increment in the nuclear size, which decreases the drive of fascination between the valence electrons and the core.
The ionization enthalpy of fluorine is considerably higher than any other halogen, which is ascribed to its little size.
Fig. 4: Ionization enthalpy of group 17 elements
Electron Gain Enthalpy
Halogens have the most extreme negative electron pick up enthalpy in the particular time frames.
The electron gain enthalpy turns out to be less negative on moving down the group.
Fluorine has less negative electron pick up enthalpy than chlorine. I.e. chlorine has the most extreme negative electron pick up enthalpy among every one of the elements. It is a result of the small size and reduced 2p sub-shell of the fluorine atom. Attributable to the small size of the fluorine particle, the approaching electron encounters a more noteworthy measure of repulsion from the electrons that are now present. The electron-electron repulsion between the approaching electron and the electrons officially introduced exceed the magnetism between the additional electron and the nucleus.
Electron Gain Enthalpy |
kJ mol-1 |
| F | -333 |
| CI | -348 |
| Br | -324 |
| I | -295 |
Electro-Negativity
The halogens have high electro-negativity values. You can see from the estimations of electro-negativity in the table that the electro-negativity diminishes slowly on moving down the group from fluorine to iodine because of the relative increase in the nuclear radii.
Fluorine, in the periodic table, is the most electronegative element.
Fig. 5: Electronegativity of group 17 elements
Physical Properties and Oxidation States
Group 17 elements are called halogens.Halogens are the most electronegative elements in the periodic table.
Physical Properties
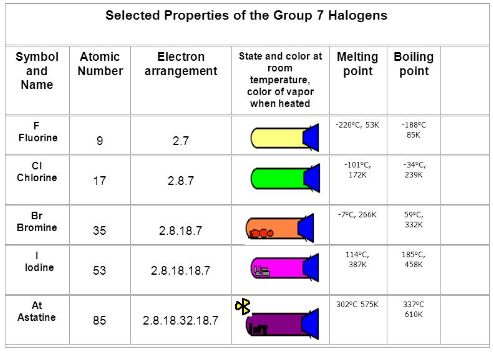
Physical properties includephysical state, color, solubility, metallic character, density, melting and boiling point, bond dissociation energy.
Physical state:The group 17 elements exist in various physical state
F,Cl are gases
Br is a liquid
I is a solid
All these are diatomic in nature.
Color: These elements displays diverse colors
F→ Pale yellow color
Cl → Greenish yellow color
Br → Reddish brown color
I → Dark violet color
Solubility: F, Cl are soluble in water
Br, I are sparingly dissolvable in water yet totally dissolvable in organic solvents.
Halogen |
Color in water |
Organic Solvent |
|
Fluorine |
Plae Yellow |
Yellow |
|
Chlorine |
Greenish Yellow |
Green |
|
Bromine |
Reddish Brown |
Brown |
|
Iodine |
Brown |
Purple |
|
Astatine |
|
|
Metallic Nature: The metallic nature increases as we move down the group.
Due to high ionization enthalpy values all these elements are non metallic in nature.
Density: The densities increases moving from F to I
Halogen |
Density |
|
Fluorine |
0.0017 |
|
Chlorine |
0.0032 |
|
Bromine |
3.1028 |
|
Iodine |
4.933 |
|
Astatine |
|
Melting and boiling points: Melting and boiling points of these elements increase regularly from Fluorine to Iodine.
Bond dissociation energy: Bond dissociation energies of these elements step by step diminishes from top to base with the exception of fluorine.
Oxidation states: General electronic configuration: ns2 np5
These elements have 7 electrons in their valence shell. They require 1 electron to finish their octet. It can be accomplished by picking up or by sharing the electron. Hence, the normal oxidation state of these elements is – 1.
These elements likewise show +1, +3, +5 oxidation states alongside - 1 oxidation state.
Exception: Fluorine shows - 1 oxidation state simply because it doesn't have any d-orbital in their valence shell.
Cl, Br &I all displays distinctive oxidation states +1, +3, +5, +7. This is because of the nearness of empty d-orbital in their valence shells.
These positive oxidation states are seen in interhalogens, oxoacids, and oxides.
Fig. 6: Selected properties of group 17 elements
Chemical Properties
Oxidizing Power
Since all halogens have a strong inclination to acknowledge electrons, they go about as great oxidizing agents. Out of the considerable number of halogens, fluorine is the most grounded oxidizing agent and can oxidize all other halide particles to halogen in a solution. As we move down the group from F to I, oxidizing power diminishes. Henceforth chlorine can oxidize bromide particle to bromine and in addition iodide particles to iodine.
Cl2 + 2Br¯ → Br2 + 2Cl¯
Cl2 + 2I¯ → I2 + 2Cl¯
Similarly, bromine can oxidize iodide particle to iodine.
Br2 + 2 I¯ → I2 + 2Br¯
Cl2(aq) |
Br2(aq) |
I2(aq) |
|
|
Cl–(aq) |
Stays yellow solution (no reaction) |
Stays brown solution (no reaction) |
|
|
Br–(aq) |
Yellow solution forms (Br2 forms) |
||
|
I–(aq) |
Brown solution forms (I2 forms) |
Brown solution forms(I2 forms) |
Despite what might be expected, halide particles act as reducing agents. Their reducing capacity diminishes from fluoride particle to iodide particle.
Reaction with Hydrogen
All halogens react with hydrogen to form acidic hydrogen halides. The acidity of these hydrogen halides reduces from HF to HI. Regardless, the reactivity of halogens towards hydrogen lessens from fluorine to iodine. Fluorine in dark reacts brutally; chlorine requires the sunshine, while bromine reacts with hydrogen just on heating. Iodine reacts with hydrogen on heating in the presence of a catalyst.
In dark
H2 + F2 → 2HF
H2 + Cl2 → 2HCl
Δ
H2 + Br2 → 2HBr
Δ
H2 + I2 → 2HI
Reaction with Oxygen
Like diverse elements, halogens in similar manner form oxides with oxygen. But, by far most of the oxides of halogen are not steady. Beside oxides, halogens also shape halogen oxoacids and oxoanions. The general condition for oxides is in the range from X2O to X2O7, while the general condition for oxoacids is in the scope of HOX to HOXO3 (there is just HOF with fluorine) and for oxoanions are shaped in the range from XO- to XO4-.
Fig. 7: Oxides of halogens
Reaction with Metals
In view of the high reactivity of halogens, they instantly react with most of the metals to form the resulting metal halides. For example, sodium reacts with chlorine gas to shape sodium chloride. Making of sodium chloride is an exothermic reaction and produces a splendid yellow light with a great deal of heat energy.
2Na(s) + Cl2(g) → 2NaCl(s)
Metal halide is ionic in nature in light of the high electro negativity of halogen and high electro positivity of metals. The ionic character of metal halides reduces from fluorine to iodine.
Reaction with other Halogens
Halogens reacts with each other to shape Interhalogen compounds. The general recipe of these compounds is XYn, where n = 1, 3, 5 or 7. In a given equation, "X" must be the less electronegative halogen contrasted with "Y".
XY |
XY3 |
XY5 |
XY7 |
|
CIF3, BrF3,IF3, ICI3 |
BrF5 |
IF7 |
Anomalous Behaviour of Fluorine
The anomalous behaviour in properties like ionization energy, bond dissociation energy, electro-negativity, electrode potentials, ionic and covalent radii, electron gain enthalpy, melting point, and boiling point is because of the small nuclear size, high electro-negativity, low bond separation energy and no accessibility of d-orbitals in the valence shell of Fluorine.
Uses of Halogens
-
Fluorine compounds are utilized as a part of toothpaste and some drinking water supplies since fluoride compounds react with teeth enamel and counteract tooth rotting.
-
Chlorine is utilized for bleaching reasons, in the metallurgy of gold and platinum, furthermore in the arrangement of natural halogen compounds.
-
Chlorine is utilized as a part of the cleansing of drinking water.
-
Since iodine kills the germs on the skin without harming the skin itself, it is utilized as an antiseptic.
Selected properties of group 17 are tabulated below:
Property |
Fluorine |
Chlorine |
Bromine |
Iodine |
Astatine |
|
Atomic Symbol |
Fluorine |
CI |
Br |
I |
At |
|
Atomic Number |
9 |
17 |
35 |
53 |
85 |
|
Atomic Mass (AMU) |
19 |
35.45 |
79.9 |
126.9 |
210 |
|
Valence Electron Configuration |
2s2 2p5 |
3s23p5 |
4s2 4p5 |
5s2 5p5 |
6s26p5 |
|
Melting Point/Boiling Point (°C) |
-220/-188 | -102/-34.0 | -7.2/58.8 | 114/84 |
302/- |
|
Density (g/cm3) at 25°C |
1.55 (g/L) |
2.90 (g/L) |
3.1 |
4.93 |
- |
|
Atomic Radius (PM) |
42 |
79 |
94 |
115 |
127 |
|
First Ionization Energy (KJ/mol) |
1681 |
1251 |
1140 |
1008 |
926 |
|
Normal Oxidation State(s) |
-1 |
-1 + (+1, +3, +5, +7) | -1(+1, +3, +5, +7) | -1(+1, +3, +5, +7) | -1, +1 |
|
Ionic Radius (PM)+ |
133 |
181 |
196 |
220 |
- |
|
Electron Affinity (kJ/mol) |
-328 |
-349 |
-325 |
-295 |
-270 |
|
Electrone Gravitiy |
4.0 |
3.2 |
3.0 |
2.7 |
2.2 |
|
Standard Reduction Potential (E°, V) (X2 → X- in the basic solution) |
+ 2.87 |
+1.36 |
+10.7 | +0.54 | +0.30 |
|
Dissociation Energy of X2(g) (kJ/mol) |
158.8 |
243.6 |
192.8 |
151.1 |
80 |
|
Product of Reaction with O2 |
O2F2 |
None |
None |
None |
None |
|
Type of Oxide |
Acidic |
Acidic |
Acidic |
Acidic |
Acidic |
|
Product of Reaction with N2 |
None |
None |
None |
None |
|
|
Product of Reaction with H2 |
HF |
HCI |
HBr |
HI |
Hat |
|
The configuration shown does not include filled d and f subshells. |
|||||
|
+The value cited are ffor the six-coordinate anion (X-) |
|||||
Watch this Video for more reference
More Readings