Compounds of the Alkaline Earth Metals
Table of Content |
General Characteristics of Compounds of the Alkaline Earth Metals
-
Oxides of Alkali Earth Metals
 The oxides of alkali earth metals (MO) are obtained either by heating the metals in oxygen or by thermal decomposition of their carbonates.
The oxides of alkali earth metals (MO) are obtained either by heating the metals in oxygen or by thermal decomposition of their carbonates.
2M + O2  2MO ( M = Be, Mg, Ca)
2MO ( M = Be, Mg, Ca)
MCO3  MO + CO2 ( M = Be, Mg, Ca, Sr, Ba)
MO + CO2 ( M = Be, Mg, Ca, Sr, Ba)
Expect BeO all other oxides are extremely stable ionic solids due to their high lattice energies.
These have high melting point, have very low vapour pressure, are very good conducts of heat, are chemically inert and act as electrical insulators. Therefore, these oxides are used for lining furnaces and hence used as refractory materials.
Due to small size of beryllium ion, BeO is covalent but still has high melting point because of its polymeric nature.
-
Hydroxides of Alkali Earth Metals
 The hydroxides of Ca, Sr & Ba are obtained either by treating the metal with cold water or by reacting the corresponding oxides with water. The reaction of these oxides with H2O is also sometimes called as slaking.
The hydroxides of Ca, Sr & Ba are obtained either by treating the metal with cold water or by reacting the corresponding oxides with water. The reaction of these oxides with H2O is also sometimes called as slaking.
M + 2H2O → M (OH)2 + H2 ( M = Ca, Sr, Ba)
MO + H2O → M (OH)2 (M = Ca, Sr, Ba)
Be(OH)2 and Mg(OH)2 being insoluble are obtained from suitable metal ion solutions by precipitation with OH- ions.
BeCI2 + 2NaOH → Be (OH)2 ↓ + 2NaCI
MgSO4 + 2NaOH → Mg (OH)2 ↓ + Na2SO4
Properties of Hydroxides of Alkali Earth Metals
(i) Basic Character
All the alkaline earth metal hydroxides are bases except Be (OH)2 which is amphoteric. This basic strength increases as we move down the group. This is because of increase in size which results in decrease of ionization energy which weakens the strength of M – O bonds in MOH and thus increases the basic strength. However, these hydroxides are less basic than the corresponding alkali metal hydroxides because of higher ionization energies, smaller ionic sizes and greater lattice energies.
(ii) Solubility in Water
Alkaline earth metals hydroxides are less soluble in water as compared to alkali metals.
The solubility of the alkaline earth metal hydroxides in water increases with increase in atomic number down the group. This is due to the fact that the lattice energy decreases down the group due to increase in size of the alkaline earth metals cation whereas the hydration energy of the cation remains almost unchanged. The resultant of two effects i.e.
ΔHsolution = ΔHlattice - ΔHHydration
Becomes more negative as we move from Be(OH)2 to Ba(OH)2 which accounts for increase in solubility.
-
Halides of Alkali Earth Metals
The alkaline earth metals combine directly with halogen at appropriate temperature forming halides MX2.
These halides can also be prepared by the action of halogen acids (HX) on metals, metals oxides, hydroxides and carbonates.
M + 2HX → MX2 + H2
MO + 2HX → MX2 + H2O
M (OH)2 + 2HX → MX2 + 2H2O
MCO3 + 2HX → MX2 + H2O + CO2
Properties of Halides of Alkali Earth Metals
-
All beryllium halides are essentially covalent and are soluble in organic solvents. They are hydroscopic and fume in air due to hydrolysis. On hydrolysis, they produce acidic solution.
BeCI2 + 2H2O → Be (OH)2 + 2HCI -
The halides of all other alkaline earth metals are ionic. Their ionic character, however increases as the size of the metal ion increase.
-
Except BeCl2 all other chlorides of group 2 form hydrates but their tendency to form hydrates decreases for eg –
MgCl2.6H2O, CaCl2.6H2O. -
The hydrated chloride, bromides and iodides of Ca, Sr and Ba can be dehydrated on heating but those of Be and Mg undergo hydrolysis.
-
BeF2 is very soluble in water due to the high hydration energy of the small Be+2ion. The other fluorides (MgF2, CaF2, SrF2 and BaF2) are almost insoluble in water. Since on descending the group lattice energy decreases more rapidly than the hydration energy. Therefore whatever little solubility these fluorides have that increase down the group.
-
The chlorides, bromides and iodides of all other elements i.e. Mg, Ca, Sr, Ba are ionic have much lower melting points than the fluorides and are readily soluble in water. The solubility decreases some what with increasing atomic number.
-
Except of BeCl2 and MgCl2, the other chlorides of alkaline earth metals impart characteristics colour to flame.
CaCl2 = Brick red colour
SrCl2 = Crimson colour
BaCl2 = Grassy green colour
Uses
-
Calcium fluoride or fluorospar (CaF2) is by far the most important of all the fluorides of the alkaline earth metals since it is the only large scale source of fluorine.
-
CaCl2 is widely used for melting ice on roads, particularly in very cold countries because 30% eutectic mixture of CaCl2/ice freezes at 218 K as compared to NaCl /ice at 255K.
-
CaCl2 is also used as a desiccant (drying agent) in the laboratory.
-
Anhydrous MgCl2 is used in the electrolytic extraction of magnesium.
Solubility and thermal stability of oxo salts
The salts containing one or more atoms of oxygen such as oxides, hydroxides, carbonates, bicarbonates, nitrites, nitrates, sulphates, oxalates and phosphates are called oxo salts.
-
Sulphates of Alkali Earth Metals
The sulphates of alkaline earth metals (MSO4) are prepared by the action of sulphuric acid on metals, metals oxides, hydroxides and carbonates.
M + H2SO4 → MSO4 + H2
MO + H2SO4 → MSO4 + H2O
M (OH)2 + H2SO4 → MSO4 + 2H2O
MCO3 + H2SO4 → MSO4 + CO2 + H2O
Properties of Sulphates of Alkali Earth Metals
The sulphates of alkaline earth metals are all white solids.
-
Solubility:The solubility of the sulphates in water decreases down the groups i.e. Be > Mg > Ca > Sr > Ba.
Thus BeSO4 and MgSO4 are highly soluble, CaSO4 is sparingly soluble but the sulphates of Sr, Ba and Ra are virtually insoluble.
Reason
The magnitude of the lattice energy remains almost constant as the sulphate is so big that small increase in the size of the cation from Be to Ba does not make any difference. However the hydration energy decreases from Be+2 to Ba+2 appreciably as the size of the cation increase down the group. Hence, the solubilities of sulphates of alkaline earth metals decrease down the group mainly due to the decreasing hydration energies from Be+2 to Ba+2. The high solubility of BeSo4 and MgSO4 is due to high hydration energies due to smaller Be+2 and Mg+2 ions. -
Stability: The sulphates of alkaline earth metal decompose on heating giving the oxides and SO3.
MSO4 MO + SO3
MO + SO3
The temperature of decomposition of these sulpahtes increases as the basicity of the hydroxide of the corresponding metal increase down the group
Magnesium Sulphate,Epsom Salt MgSO4.7H2O
Magnesium sulphate occurs as kieserite MgSO4.H2O in Stassfurt (Germany) deposit or as Epsom salt in the mineral water of the Epsom springs in England.
Preparation of Magnesium Sulphate
(i) From dolomite
The dolomite ore is boiled with dil. H2SO4:
CaCO3.MgCO3 + 2H2SO4 → CaSO4 ↓ + MgSO4 + 2H2O + 2CO2
The ppt of calcium sulphate are filtered off and the solution on concentration and cooling gives crystals of MgSO4.7H2O.
(ii) From Magnesite
The magensite ore is powdered and dissolved in dilute H2SO4. The resulting solution is concentrated and cooled when crystals of MgSO4.7H2O separate out.
MgCO3 + H2SO4 → MgSO4 + H2O + CO2
(iii) From Kieserite
The mineral Kieserite (MgSO4.H2O) is powdered and dissolved in water. The resulting solution upon concentration and cooling gives crystals of MgSO4.7H2O.
(iv) Laboratory Preparation
In the laboratory MgSO4 is prepared by dissolving Mg metal or MgO or MgCO3 with dilute H2SO4.
Mg + H2SO4 → MgSO4 + H2
MgO + H2SO4 → MgSO4 + H2O
MgCO3 + H2SO4 → MgSO4 + CO2 + H2O
The resulting solution upon concentration and cooling gives crystals of MgSO4.7H2O.
Properties Magnesium Sulphate
It is deliquescent and readily dissolves in water. Hydrates with 12, 6 and 1 molecule of water of crystallisation are also known. All these hydrates are converted into the anhydrous salt, when heated to 200°C and on further heating they decompose to form the oxide. Magnesium sulphate gives rise to double salt with the alkali sulphate.
-
Magnesium sulphate is a colourless efflorescent crystalline solid highly soluble in water.
-
Isomorphism: MgSO4.7H2O is isomorphous with ZnSO4.7H2O & FeSO4.7H2O compounds having same crystal structure are called isomorphous and the phenomenon is called Isomorphism.
-
Action of Heat: When heated it losses 6 molecules of water to give Magnesium sulphate monohydrate which becomes anhydrous when heated to 503 K and finally decomposes to MgO & SO3 gas on strong heating.
MgSO4. 7H2O![\xrightarrow[-6H_2O]{423K}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_xrightarrow-6h_2o423k.jpg) MgSO4 H2O
MgSO4 H2O ![\xrightarrow[-H_2O]{503K}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_xrightarrow-h_2o503k.jpg) MgSO4
MgSO4 ![\xrightarrow[Strong]{Heating}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_xrightarrowstrongheating.jpg) MgO + SO3
MgO + SO3
Uses of Magnesium Sulphate
-
MgSO4 is used as purgative medicine.
-
It is used as mordant for cotton in dyeing industry.
-
It is used in preparation of fire proof textile and wood.
-
Anhydrous MgSO4 is used as a drying agent in organic chemistry.
-
It is used in preparation of platinised asbestors which is used as a catalyst in the contact process for the manufacture of H2SO4.
Illustrations. |
|
Question: The salts having the similar crystal structure are called isomorphous salts. Examples: MgSO4.7H2O, ZnSO4.7H2O, FeSO4.7H2O |
Oxides of Magnesium and Calcium
-
MgO (Magnesia)
It is made by heating magnesite (MgCO3).
MgCO3 → MgO + CO2
It is very slightly soluble in water imparting an alkaline reaction to the solution.
MgO + H2O → Mg(OH)2
-
Calcium oxide, Quick lime CaO
(i) Preparation
It is made by decomposing limestone at high temperature about 1000o C
CaCO3  CaO + CO2; ΔH = + 179.9 KJ
CaO + CO2; ΔH = + 179.9 KJ
The temperature should not be raised above 1270 K. Otherwise silica present as impurity in lime will combine with calcium oxide to form infusible calcium silicate.
CaO + SiO2  CaSiO3
CaSiO3
(ii) Properties
-
It is white amorphous powder, which emits intense white light (lime light), when heated in the oxy-hydrogen flame.
-
It reacts with strongly heated silica,forming easily fusible calcium silicate.
CaO + SiO2 → CaSiO3 -
CaO reacts with water evolving huge amount of heat and produce slaked lime.
CaO + H2O → Ca(OH)2
-
Action of acids and acidic oxides : It is a basic oxide and hence combines with acids and acidic oxides forming salts.
CaO + 2HCl → CaCl2 + H2O
CaO + SO2 → CaSO3 -
Reaction with coke: When heated with coke in electric furnace at 2273 – 3273 K, it forms calcium carbide.
CaO + 3C CaC2 + Co
CaC2 + Co -
Reaction with ammonium salt
On heating with ammonia salts, it liberates ammonia gas.
?CaO + 2NH4Cl → CaCl2 + 2NH3 + H2O
(iii) Industrial uses of lime and Limestone
Calcium oxide is called lime or quick lime. It main industrial uses are
-
It is used in steel industry to remove phosphates and silicates as slag.
-
It is used to make cement by mixing it with silica, alumina or clay.
-
It is used in making glass.
-
It is used in lime soda process for the conversion of Na2CO3 to NaOH & vice versa.
-
It is used for softening water, for making slaked lime Ca(OH)2 by treatment with water and calcium carbide CaC2.
Illustration |
|
Why does a piece of burning magnesium continue to burn in SO2? Solution: This is because the reaction of Mg with SO2 is exothermic. 2Mg + SO2 → 2MgO + 1/8S8 + Heat |
Hydroxides of Mg & Ca
-
Magnesium Hydroxide [Mg(OH)2]
It is obtained by adding caustic soda solution to a solution of magnesium sulphate or chloride.
MgSO4 + 2NaOH → Na2SO4 + Mg(OH)2
Properties of Magnesium Hydroxide
-
It is converted into its oxide on heating.
Mg(OH)2 → MgO + H2O -
It dissolves in NH4Cl solution easily.
Mg(OH)2 + 2NH4Cl → MgCl2 + 2NH4OH
-
Calcium Hydroxide, Slaked lime [Ca(OH)2]
(i) Preparation of Calcium Hydroxide
-
From Quick lime: Calcium hydroxide is prepared on commercial scale by adding water to quick lime (Slaking of lime)
CaO + H2O → Ca (OH)2
During the process of slaking, lumps of quick lime crumble to a fine power. -
From calcium chloride: It is obtained by treating calcium chloride with caustic soda.
CaCl2 + 2NaOH → Ca (OH)2 + 2NaCI
(ii) Physical Properties Calcium Hydroxide
It is a white amorphous powder sparingly soluble in water, the solubility decreasing further with rise in temperature. An aqueous solution is known as lime water and a suspension of slaked lime in water is called milk of lime.
(iii) Chemical Properties Calcium Hydroxide
- Action of heat: It loses water only at temperature above 700 K.
Ca(OH)2 CaO + H2O
CaO + H2O
- Reaction with chlorine: It forms calcium hypochlorite a constituent of bleaching power.
2Ca (OH)2 + 2Cl2 → CaCl2 + Ca (OCl)2 + 2H2O
-
Reaction with carbon dioxide: When CO2 is passed through lime water, it turns milky due to formation of insoluble calcium carbonate
Ca(OH)2 + CO2 → CaCO3 ↓ + 2H2O
If excess of CO2 is passed CaCO3 (ppt) dissolves to form soluble calcium bicarbonate due to which milkiness disappears.
CaCO3 + CO2 + H2O → Ca (HCO3)2
If this clear solution of calcium bicarbonate is heated, the solution again turns milky due to the decomposition of ca(HCO3)2 back to CaCO3.
Ca(HCO3)2 (aq) → CaCO3 (s) + CO2 (g) + H2O (l) -
Reaction with acids: Slaked lime being a strong base reacts with acids and acidic gases forming salts.
Ca (OH)2 + 2HCl → CaCl2 + H2O
Ca (OH)2 + SO3 → CaSO4 + H2O
However, Ca(OH)2 does not dissolve in dil. H2SO4 because the CaSO4 formed is sparingly soluble in water.
(iv) Uses of Slaked lime [Ca(OH)2]
-
(Slaked lime is used as a building material in form of mortar. It is prepared by mixing 3 – 4 times its weight of sand and by gradual addition of water. Its sets into a hard mass by loss of H2O and gradual absorption of CO2 from air.
-
In manufacture of bleaching powder by passing Cl2 gas.
-
In making glass and in the purification of sugar and coal gas.
-
It is used in softening of hard water.
Illustration. |
|
Which is the weakest base among NaOH, Ca(OH)2, KOH and Be(OH)2 Solution: Be(OH)2 is weakest base , because alkali metal hydroxides are more stronger base than alkaline earth metal hydroxides. Also basic character of hydroxides of alkaline earth metals increases down the group. So Be(OH)2 is the weakest one. |
-
Calcium Carbonate (CaCO3)
It occurs in nature as marble, limestone, chalk, coral, calcite, etc. It is prepared as a white powder, known as precipitated chalk, by dissolving marble or limestone in hydrochloric acid and removing iron and aluminium present by precipitating with NH3, and then adding ammonium carbonate to the solution; the precipitate is filtered, washed and dried.
CaCl2 + (NH4)2CO3 →CaCO3+2NH4Cl
(i) Properties of Calcium Carbonate
It dissolves in water containing CO2, forming Ca(HCO3)2 but is precipitated from solution by boiling.
CaCO3 + H2O + CO2 →Ca(HCO3)2
Illustrations. |
|
Question : Thermal decomposition of a compound 'X' yields, a basic oxide ( Y ) and acidic oxide( Z ) simultaneously. The acidic oxide(Z) can be absorbed by alkaline KOH. What is X,Y,Z.. Solution: CaCO3 (X) → CaO (Y) + CO2 (Z) CO2 + 2KOH → K2CO3 +H2O |
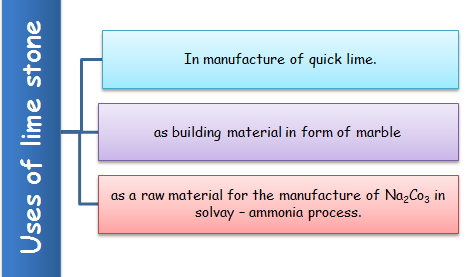
(ii) Uses of lime stone (CaCO3)
-
It is used as building material in form of marble.
-
It is used as a raw material for the manufacture of Na2Co3 in solvay – ammonia process.
-
Commercial limestone contains iron oxide, alumina, magnesia, silica & sulphur with a CaO content of 22 – 56% MgO content upto 21%. It is used as such as a fertilizer
.
Magnesium Carbonate (MgCO3)
It is obtained as magnesite in nature. It can be prepared as a white precipitate by adding sodium bicarbonate to a solution of a magnesium salt.
MgCl2 + NaHCO3 → MgCO3 + NaCl + HCl
Properties of Magnesium Carbonate
-
It is very much more soluble in water.
-
It dissolves in water containing CO2 due to formation of soluble bicarbonate.
MgCO3 + H2O + CO2 → Mg(HCO3)2
Bicarbonates of Mg & Ca
-
Calcium bicarbonate [Ca(HCO3)2]
It is obtained when CaCO3 is dissolved in water containing CO2 but it remains in the solution form CaCO3 + H2O + CO2 → Ca(HCO3)2.
-
Magnesium bicarbonate [Mg(HCO3)2]
It is obtained when MgCO3 is dissolved in water containing CO2 but it remains in the solution form MgCO3 + H2O + CO2 → Mg(HCO3)2.
Halides of Mg & Ca
-
Calcium Chloride (CaCl2×6H2O)
It separates out as deliquescent crystals when a solution of lime or calcium carbonate in HCl is evaporated.
CaCO3 + 2HCl → CaCl2 + H2CO3
But it separates out from the reaction mixture as CaCl2×6H2O. The anhydrous salt is obtained on heating above 200°C.
Properties of Calcium Chloride
It is a colourless, deliquescent salt, highly soluble in water. The anhydrous salt is an excellent drying agent.
-
Magnesium Chloride (MgCl2×6H2O)
It is prepared in the laboratory by crystallizing a solution of the oxide, hydroxide or carbonate in dilute hydrochloric acid.
MgO + 2HCl → MgCl2 + H2O
Properties
It is colourless, crystalline salt, deliquescent in nature and exceedingly soluble in water.
Illustrations. |
|
Question : Complete the following reactions: (i) MgCl2. 6H2O Solution: (i) MgCl2. 6H2O (ii) MgCl2. 6H2O |
Plaster of Paris, CaSO4.1/2 H2O or (CaSO4)2.H2O
It occurs in nature as gypsum and the anhydrous salt as anhydride. It is prepared by precipitating a solution of calcium chloride or nitrate with dilute sulphuric acid.
The effect of heat on gypsum or the dihydrate presents a review of interesting changes. On heating the monoclinic gypsum is first converted into orthorhombic form without loss of water. When the temperature reaches 120°C, the hemihydrate or plaster of paris is the product. The latter losses water, becomes anhydrous above 200°C and finally above 400°C, it decomposes into calcium oxide.
2CaSO4 → 2CaO + 2SO2↑ + O2↑
The following conditions are necessary
-
The temperature should not be allowed to rise above 393 K because above this temperature the whole of water of crystallization is lost. The resulting anhydrous CaSO4 is called dead burnt plaster because it does not have the properties of setting with water.
-
The gypsum should not be allowed to come in contact with carbon containing fuel otherwise some of it will be reduced to calcium sulphite.
(i) Properties of Plaster of Paris
It is a white powder. On mixing with 1/3rd its weight of water, it forms a plastic mass which sets into a hard mass of interlocking crystals of gypsum within 5 to 15 minutes. It is due to this reason that it is called plaster. The addition of common salt accelerates the rate of setting, while a little borax or alum reduces it. The setting of plaster of paris is believed to be due to rehydration and its reconversion into gypsum.
2CaSO4. 1/2 H2O + 3H2O → 2CaSO4. 2H2O
Plaster of Paris gypsum
(ii) Uses of Plaster of Paris:
-
Plaster of pairs is used for producing moulds for pottery and ceramics & casts of statues & busts.
-
It is used in surgical bandages used for plastering broken or fractured bones.
-
It is also used in dentistry.

Question 1: Formula of gypsum is
a. CaSO4. 2H2O
b. CaSO4. 1/2 H2O
c. CaSO4. H2O
d. CaSO4. 3/2 H2O
Question 2: Which of the following statements is incorrect?
a. Expect BeO all other oxides of alkali earth metals are extremely stable ionic solids due to their high lattice energies
b. Plaster of pairs is used for producing moulds for pottery and ceramics & casts of statues & busts.
c. Slaked lime being a strong base reacts with acids and acidic gases forming salts.
d. Be(OH)2 and Mg(OH)2 are highly soluble in water.
Question 3: CaCO3 + 2HCl →
a. CaCl2 + H2CO3
b. CaCl2 + CO2
c. Ca(OH)2 + Cl2
d. CaH2 + H2CO3
Question 4: Which of the following halides do not impart colour to the flame?
a. CaCl2
b. SrCl2
c. BaCl2

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
d |
a |
d |
Related Resources
-
Look here for past year papers of IIT JEE
-
You should also refer to the syllabus of chemistry for IIT JEE
-
You can also refer to alkali metals
To read more, Buy study materials of S- Block elements comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.