Redox Reactions
Table of Content |
Redox Reactions

 Chemical Reaction is the process which leads to the transformation of one set of chemical substances to other substances.
Chemical Reaction is the process which leads to the transformation of one set of chemical substances to other substances.
Classically, chemical reactions encompass changes that strictly involve the motion of electrons in forming and breaking of the chemical bonds.
The concept of electron transfer can easily explain the redox reaction in case of ionic substances. However, for covalent compounds oxidation and reduction or redox reactions are explained using a new term oxidation number .
The term oxidation was first used to mean the addition of oxygen to an element or compound, or the removal of hydrogen from a compound. Reduction meant the addition of hydrogen to an element or compound, or the removal of oxygen from a compound. Such definitions have been extended and now-a-days many oxidation-reduction, or redox, reactions are best interpreted in terms of transfer of electrons.
-
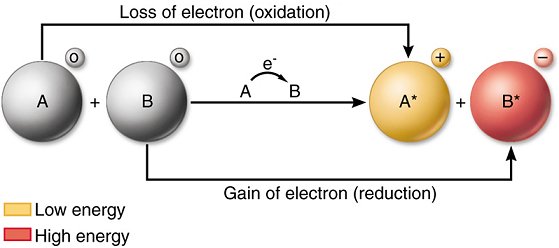
Oxidation is defined as the loss of electrons by a chemical species (atom, ion or molecule).
-
Reduction is the gain of electrons by a chemical species (atom, ion or molecule).
-
An oxidising agent that chemical species which takes electrons thus it is an electron acceptor.
-
A reducing agent is the chemical species that gives electrons and thus acts as an electron donor.
When Fe2+(aq) ions are being oxidised they are acting as reducing agents, and when Fe3+(aq) ions are being reduced they are acting as oxidising agents. In general ;

We will gain in depth knowledge of redox reactions under following sub topics
What are Redox Reactions?
-
Redox reactions are those chemical reactions in which both oxidation as well as reduction occur simultaneously.
-
Oxidation and reductions go hand in hand.
-
The substance which undergo reduction is called oxidising agent while the substance which undergo oxidation is called reducing agent. One can say that the substance that causes the oxidation of any substance in reaction is called the oxidizing agent while the substance that causes the reduction is called the reducing agent.
-
Neither reduction nor oxidation occurs alone. Both of them occur simultaneously. Since both these reactions must occur at the same time they are often termed as "redox reactions". The oxidation or reduction portion of a redox reaction, including the electrons gained or lost can be determined by means of a Half-Reaction
Watch this Video for more reference
Types of Redox Reactions
Redox reactions are divided into two main types.
(i) Inter molecular Redox Reactions:
In such redox reactions, one molecule of reactant is oxidized whereas molecule of other reactant is reduced.

(ii) Intra molecular Redox Reactions:
One atom of a molecule is oxidized and other atom of same molecule is reduced then it is intra molecular redox reaction.

Basic Terms Used
Molecular Equations
When the reactant and products involved in a chemical change are written in molecular form in a chemical equation, it is termed as molecular equation.
Example: MnO2 + 4HCl → MnCl2 + 2H2O +Cl2
In above example the reactant and products have been written in molecular forms, thus it is a molecular equation.
Ionic Equation
When the reactant and products involved in a chemical change are ionic compounds, these will be present in the form of ions in the solution. The chemical change is written in ionic forms in the chemical equation, it is termed as ionic equation.
Example: MnO2 + 4H++ 4Cl- → Mn2++ 2Cl- + 2H2O +Cl2
In the above example, the reactant and products have been written in ionic forms, thus the equation is termed as ionic equation.
The rules to be followed for writing ionic equations are:
-
All soluble ionic compounds involved in a chemical changes are expressed in ionic symbols and covalent substances are written in molecular form. H2O, NH3, NO2, NO, SO2, CO, CO2, etc., are expressed in molecular form.
-
The ionic compound which is highly insoluble is expressed in molecular form.
-
The ions which are common and equal in number on both sides, i.e., spectator ions, are cancelled.
-
Besides the atoms, the ionic charges must also be balanced on both the sides.
Spectator Ions
Species that are present in the solution but not take part in the reaction and are also omitted while writing the net ionic equation are called spectator ions or bystander ions.
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
In this reaction, ions are omitted and are called as spectator ions and appear on the reactant as well as product side.”
Oxidising Agent
The substance (atom, ions or molecules) that gain electrons and is thereby reduced to a low valency state is called an oxidising agent.
Reducing Agent
The substance that loses electrons and its valency thereby oxidised to a higher valency state is called a reducing agent.

Question 1: Which of the following statements regarding redox reactions is incorrect?
a. Oxidation is the process of loss of electrons by a chemical species ( atom, ion or molecule).
b. Reduction is the process of gain of electrons by an atom, ion or molecule.
c. A reducing agent is one that undergo reduction
d. A reducing agent gives electrons;
Question 2: Which of the following rules to be followed for writing ionic equations is incorrect?
a. All soluble ionic compounds involved in a chemical changes are expressed in ionic symbols and covalent substances are written in molecular form. H2O, NH3, NO2, NO, SO2, CO, CO2, etc., are expressed in molecular form.
b. The ionic compound which is highly insoluble is expressed in molecular form.
c. The ions which are common and equal in number on both sides are written on product side.
d. Besides the atoms, the ionic charges must also be balanced on both the sides.
Question 3: Which of the following equations is not a redox reaction?
a. Fe2+ → Fe3+ + e-
b. MnO2 + 4HCl → MnCl2 + 2H2O +Cl2
c. Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
d. MnO2 + 4H++ 4Cl- → Mn2++ 2Cl- + 2H2O +Cl2
Question 4: In the reaction MnO2 + 4HCl → MnCl2 + 2H2O +Cl2
Which of the following species acts as reducing agent?
a. MnO2
b. HCl
c. Cl-

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
c |
c |
a |
b |
Related Resources
-
Click here to know the syllabus of chemistry for IIT JEE
-
You can also have a look at past year papers of IIT JEE
To read more, Buy study materials of Redox Reactions comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.