Emission of Electrons
Table of Content |
Electron emission refers to the process of discharge of electrons from the surface of a metal. At room temperature the free electrons move randomly within the conductor, but they don’t leave the surface of the conductor due to attraction of positive charges. Some external energy is required to emit electrons from a metal surface. Thus, at the surface of the metal, a free electron is stopped by various forces which prevent it from leaving the metal. The metallic surface hence acts as a barrier to the free electrons whose kinetic energy increases and is hence called the surface barrier. If the appropriate amount of energy is provided to the free electrons then their kinetic energy rises and in such a case, the electrons may even cross the surface barrier to leave the metal.
Work Function (W)
Work function is a property of the metallic surface. The minimum amount of energy required by an electron to vanish or escape from the surface of the metal is called the work function. The work function of pure metals varies (roughly) from 2eV to 6eV. Its value varies according to the nature and the purity of the metal and its defined as,

Here h is the Planck’s constant and  is the threshold frequency.
is the threshold frequency.
The energy required to liberate an electron from metal surface may arise from various sources such as heat, light, electric field etc. Depending on the nature of source of energy, the following methods of emission of electron are possible:
Thermionic Emission
This is one of the ways of providing energy to the metal to emit electrons from its surface. The emission of electron forces is achieved by heating the electrode. As a result of heating, the electrons get sufficient amount of energy that they liberate from the surface of the metal. The number of electrons emitted depends on the temperature. Higher the temperature, greater is the number of emissions. The electrons so emitted are called the thermions. Such a method is generally exercised in vacuum tubes.
Field Emission
Emission of electrons from the surface of a metal by putting it under a strong electric field is called field emission. When the metal is subjected to an electric field the free electrons present in the material face an electric force in the direction opposite to that of the field. As a result beyond a point the electrons start exiting from the metal surface.
As stated earlier, in the absence of a strong electric field, an electron must acquire the minimum energy termed as the work function in order to escape from the surface which hinders the passage of electrons. When the material is placed in an electric circuit, the work function is reduced to such a level that some electrons get the sufficient amount of energy to get through the surface barrier. Hence, the emission of electrons from the surface as a result of electric current is termed as field emission. This holds true in the case of ions as well as they may also be emitted from a solid subjected to a high electric field.
Secondary Emission
Secondary emission is the phenomenon in which the primary particles with sufficient energy when striking a surface or while passing through a material induce the release of secondary particles. The primary particles are generally the charged particles like the ions or electrons. The process is termed as the secondary electron emission when the secondary particles are electrons. Similarly, when the secondary particles are ions, the process is termed as secondary ion emission. Hence, the emission or release of electrons from a metal surface by the bombardment of high speed electrons or other particle is known as secondary emission.
Photoelectric Emission
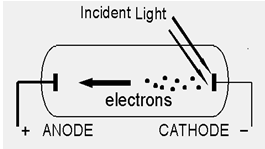
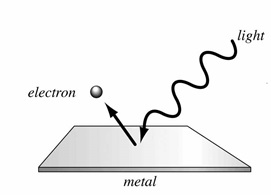
Emission of free electrons from a metal surface by falling light (or any other electromagnetic wave which has energy greater than the work function of the metal) is called photoelectric emission. The electrons so emitted are called photoelectrons. This is explained in detail as under.
When a beam of light strikes the surface of a cathode which is generally made up of potassium and sodium, the energy of the photons of light is transferred to the free electrons of the cathode. The emission or discharge of electrons takes place only when the energy of the incident photon is greater than the free electrons of the metal. The Photoelectric emission varies according to the intensity of light beam falling on the metal surface. The greater is the intensity of light beam falling on the surface, higher is the emission. This emission is employed in photo tubes which lay the basis of sound films and televisions.
Problem (JEE Advanced)
Light of wavelength 330 nm falling on a piece of metal ejects electrons with sufficient energy which requires voltage V0 to prevent them from reaching a collector. In the same setup, light of wavelength 220 nm, ejects electrons which require twice the voltage V0 to stop them in reaching a collector. The numerical value of voltage V0 is (15/x) V. Find value of x.
Solution:
Let W be the work function of metal. Then
eV0 = (hc/330 10-9) – W …...(1)
10-9) – W …...(1)
e(2V0) = (hc/220 10-9) – W …...(2)
10-9) – W …...(2)
Solving these two equations, we get,
V0 = (109 h
h c)/110
c)/110 e
e 6)
6)
= (109 6.7
6.7 10-34
10-34 3
3 108)/(110
108)/(110 1.6
1.6 10-19
10-19 6)
6)
= 15/8 volt
From the above observation we conclude that, the value of x will be, 8.
View the following video for more on photoelectric emission
|
|


Question 1:-
The concepts of a particle and a wave
(a) are clear and completely distinct from one another in both classical and modern physics.
(b)can both be applied to electromagnetic radiation.
(c)have found little use in quantum physics.
(d)all of the above are true.
Question 2:-
Emission spectra and absorption spectra
(a) for a single element complement one another.
(b) can be used to identify elements in unknown samples, but only if the element is already known by classical chemical means.
(c) when combined together form a series of bright lines.
(d) for certain pairs of closely-related elements are identical.
Question 3:-
When the sun's spectrum was first studied in detail
(a) it was discovered that the sun's interior is cooler than the exterior.
(b) very few elements are found in the sun, and all of them were well known from earth samples.
(c) an apparently new element was discovered, subsequently named helium.
(d) spectral series were found to be lacking in pure sunlight.
Question 4:-
Which of the following types of radiation is emitted directly by the electronic structures of atoms?
(a) beta radiation
(b) visible light
(c) alpha radiation
(d) gamma rays
Question 5:-
An atom is said to be in an excited state when it has one or more electrons
(a) at rest.
(b) inside the nucleus.
(c) in its lower energy level.
(d) in a larger orbit than the smallest possible orbits.


| Q.1 | Q.2 | Q.3 | Q.4 | Q.5 |
|
b |
a |
c |
b |
d |
Related Resources
-
You might like to refer Laws of Photoelectric Emission.
-
For getting an idea of the type of questions asked, refer the Previous Year Question Papers.
-
Click here to refer the most Useful Books of Physics.
- To get answer to any question related to emission of electrons click here.