Some Applications of d- and f- Block Elements
Table of Content |
Applications of the d- block Elements
-
Iron and its amalgam, steel, are utilized broadly in the development industry.
-
Titanium is utilized as a part of the manufacture of air ship and spaceship.
Fig. a: A plane made of titanium
-
Tungsten is utilized in making electrical fibres.
-
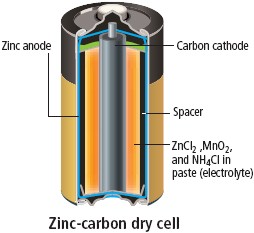
Manganese dioxide is utilized as a part of dry battery cells.
-
Zinc is utilized as the negative anode in fixed dry batteries.
Fig. b: Zinc- carbon dry cell
-
Niobium composites are utilized as a part of fly motors.
-
Tantalum is utilized to make expository weights.
-
Silver bromide is utilized as a part of photography.
-
Many d- block or transition metals and their compounds are utilized as impetuses in the chemical reactions.
Example
Ziegler-Natta, a complex of trimethyl aluminium and titanium tetrachloride are utilized in the polymerisation of ethene to polythene.
Fig. c: Zeigler Natta catalyst
Iron is utilized in the production of ammonia in Haber's process.
Fig. d: Utilization of iron in Haber’s process
Palladium chloride used in the Wacker process of oxidation of ethane to ethanol.
Applications of f-block Elements
-
Lanthanide alloys (mischmetal) utilized for the creation of instrumental steels and heat resistance.
-
Carbides, Borides, and nitrides of lanthanides are utilized as refractories.
-
Lanthanide oxides are utilized in cleaning glass as abrasives.
-
Thorium is utilized as a part of cancer treatment and in glowing gas mantles.
Fig. e: Thorium lanterns
-
Uranium is utilized as an atomic fuel.
Fig. f: Uranium as a fuel element
Fig. g: Fat Man: Implosion-Type Bomb 3D cut-away
Frequently Asked Questions (FAQs)
Q1. Which elements are known as d Block Elements?
Sol. The elements having partially filled d-orbitals in either ground state or in at least one of their ions are termed d-block elements or outer transition elements. The d-block elements are found amidst the period table.
Fig. h: d- block elements
Q2. What are the properties of d- block elements?
Sol. The elements with halfway filled d-subshell are named as D-block elements. They are additionally named as transition elements. The inadequate subshells incorporate the (n-1) d subshell. All the d-block elements have a similar number of electrons in the peripheral shell. Henceforth, they demonstrate comparable chemical properties.
The transition elements can be either regular transition elements or the non- typical transition elements. Elements of II B group Zn, Cd and Hg have a totally filled (n-1) d subshell and henceforth, can't be incorporated into the D-block. Be that as it may, they indicate properties, for example, development of complexes with ligands, for example, ammonia, amines and halide particles and are consequently, examined alongside d-block elements. Essentially, the elements of group III-A Sc, Y, La, and Ac vary from other d-block elements, however, have a not entirely filled (n-1)d subshell and are consequently, considered alongside d-block elements.
These elements that is, elements of group II B Zn, Cd and Hg and III A Sc, Y, La and Ac are named as non- typical transition elements and the other transition elements are named as typical transition elements.
The d-block incorporates three arrangements each of ten elements. These arrangements are described by the totally filled 3d, 4d, and 5d subshells and are named as 3d-(first series) Sc - Zn, 4d arrangement (second series) Y-Cd and the 5d arrangement (third series) La-Hg separately. There is a deficient fourth series comprising of just three elements in particular Ac, Ku, and Ha. In these elements, the 6d subshell begins to fill at Ac.
Q3. Which transition metals can be used to make magnets?
Sol. Iron, cobalt and nickel
Q4. Where are the transition metals found in periodic table?
Sol. The 38 elements in groups 3 through 12 of the periodic table are called "Transition Metals." As with all metals, the transition elements are both malleable and ductile, and are good conductors of electricity and heat.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free