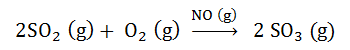Surface Chemistry
Table of Content |
|
|
What is Surface Chemistry
The study of surfaces or interface is known as Surface Chemistry.
Adsorption
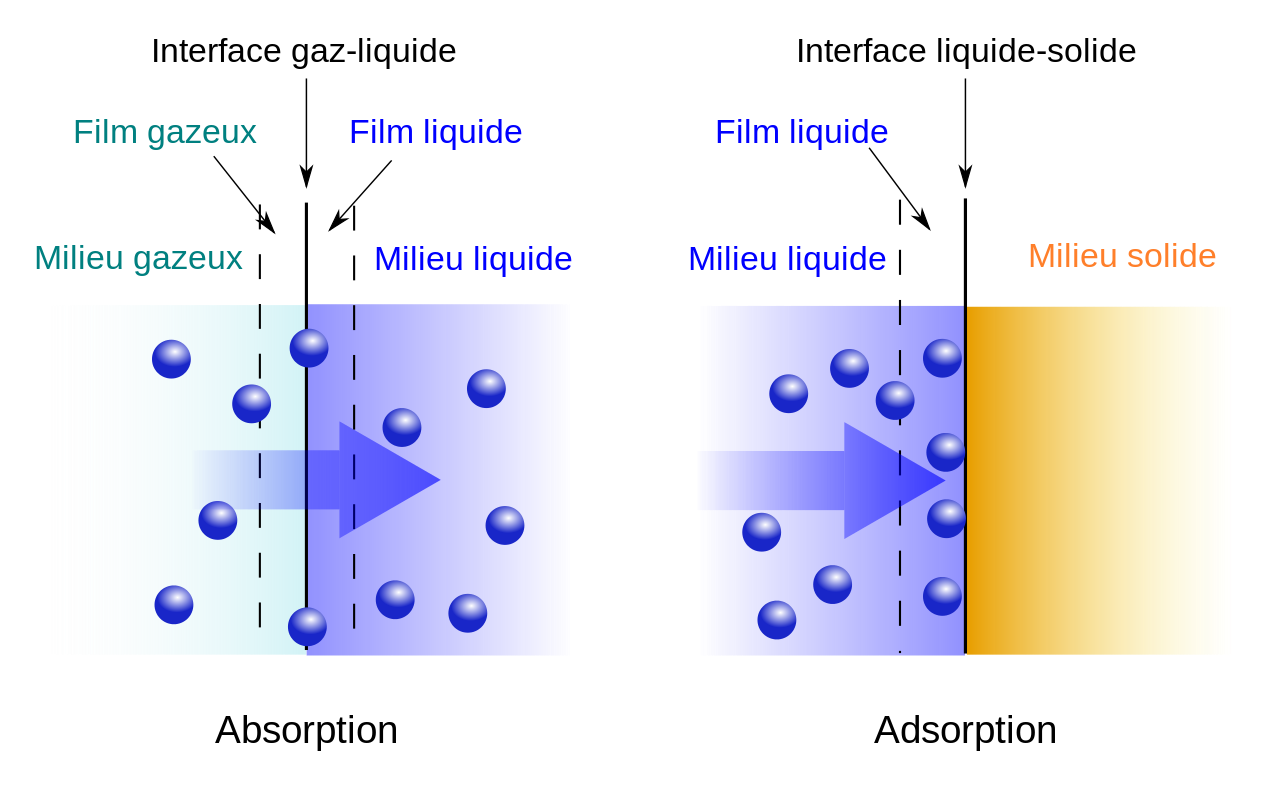
 Adsorption is defined as the deposition of molecular species onto the surface. The molecular species that gets adsorbed on the surface is known as Adsorbent and the surface on which adsorption occurs is known as Adsorbate. Common examples of adsorbents are clay, silica gel, colloids, metals etc.
Adsorption is defined as the deposition of molecular species onto the surface. The molecular species that gets adsorbed on the surface is known as Adsorbent and the surface on which adsorption occurs is known as Adsorbate. Common examples of adsorbents are clay, silica gel, colloids, metals etc.
It is a surface phenomenon. The process of removal of adsorbent from the surface of adsorbate is known as Desorption.
Fig. 1. Adsorption and Absorption
Difference between Absorption and Adsorption
Absorption |
Adsorption |
|
Substance penetrates the surface |
|
|
It occurs at uniform rate |
Rate increases initially then it decreases |
|
It is unaffected by temperature |
|
|
It is an endothermic process |
It is an exothermic process |
Types of Adsorption
There are two types of adsorption – Physical Adsorption or Physiosorption and Chemical Adsorption or Chemisorption.
Physical Adsorption
It involves adsorption of gases on solid surface via weak vander Waal’s forces.
Characteristics of Physical Adsorption:
-
There is no specificity in case of physical adsorption.
-
Physical adsorption is reversible in nature.
-
More surface area more is the rate of adsorption.
-
It is an exothermic process.
-
No activation energy is needed.
Chemical Adsorption or Chemisorption
When the gas molecules or atoms are held to the solid surface via chemical bonds, this type of adsorption is chemical adsorption or chemisorption.
Characteristics of Chemical Adsorption:
-
This type of adsorption is specific as compared to physical adsorption.
-
Chemical adsorption is irreversible.
-
Due to chemical bond formation enthalpy of chemisorption is high.
-
Activation energy is needed.
Adsorption Isotherms
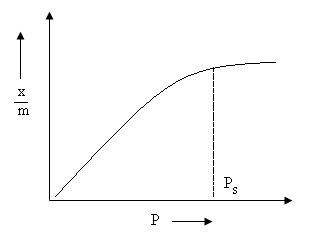
Adsorption Isotherm is a graph or a relation between the amounts of adsorbate adsorbed on the surface of adsorbent and pressure at a constant temperature.
Different adsorption isotherm was studied by different scientists:
Freundlich Adsorption Isotherm
Freundlich proposed an empirical relationship between amount of gas adsorbed by unit mass of adsorbent and pressure at a particular temperature. Following equation was proposed for freundlich adsorption isotherm:
x/m= k. p1/ n
where (n > 1)
Fig. 2. Adsorption Isotherm
x is the mass of the gas adsorbed
m is the mass of the adsorbent
p is the pressure
k and n are constants which depends on the nature of the adsorbent and the gas at a particular temperature.
Taking log of the above equation, the following equation will be observed
log x/m = log k +1/n log p
x/m is plotted on y axis and log p is on x axis. If straight line is observed then only freundlich isotherm is verified.
Slope gives 1/n and intercept gives log k. The value of 1/n varies from 0 to 1.
If 1/n is 0, adsorption is independent of pressure.
If 1/n is 1, adsorption changes with pressure.
Applications of Adsorption
-
High vacuum can be created using adsorption strategy.
-
Gas masks used in coal mines are based on adsorption principle.
-
Noble gases can be separated based on adsorption.
-
Adsorption of drugs are used to kill germs.
-
Chromatographic analysis is done on the basis of adsorption.
Catalysis
Potassium chlorate decomposes into potassium chloride and oxygen at 653-873K. But if little manganese dioxide is added, rate of decomposition occurs fast and at lower temperature range 473- 633 K. Manganese dioxide remains unchanged in terms of mass and composition. The term used for such substance is known as Catalyst. Thus, during decomposition of potassium chlorate, manganese dioxide acts as catalyst.
Types of Catalysis
Homogenous catalysis is defined as, when catalyst and reactants are in same phase, that is, either in liquid or gas.
Fig. 4. Example of Homogenous Catalysis
Heterogenous catalysis is observed when catalyst and reactants are in different phases.
Fig. 5. Examples of Heterogenous Catalysis
Important Features of Catalyst:
-
Activity is one of the important feature of catalyst. The reactants should be able to adsorb strongly on the substrate. But the adsorption should not be so strong that it will leads to the immobilization of catalyst.
-
Catalyst are selective in nature. Selectivity of different catalysts for same reactants is different. Same reactants with different catalyst gives different products.
Shape Selective Catalysis by Zeolites
When a particular catalytic reaction depends on the pore shape of the catalyst and also on size of reactants and products, such type of catalysis is known as shape-selective catalysis.
Zeolites have honeycomb like structure. They are known as Aluminosilicates. It is a three- dimensional network of silicates in which some silicon atoms are replaced by aluminum atoms. They are widely used in petrochemical industries such as for the cracking of the hydrocarbons and isomerization.
Enzyme Catalysis
They are complex organic molecules produced by plants and animals. They are commonly known as biochemical catalyst. Different enzymes catalyze different reactions. For Example, invertase converts sucrose into glucose and fructose.
Characteristics of Enzyme Catalysis
-
Enzyme catalyzed reactions are very efficient.
-
These enzymes are active at a particular range of temperature.
-
They work actively under optimum pH.
-
Presence of activators increases their activity whereas presence of inhibitors inhibits their activity.
Colloids
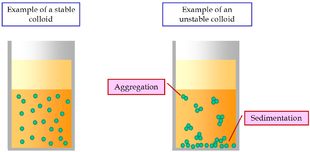
Colloids are defined as heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance known as Dispersion Medium.
Fig. 7. Colloids
Properties of Colloidal Solution
-
They form bigger aggregates as compared to true solutions. Colligative properties such as osmotic pressure, lowering in vapor pressure etc. are of small order as compared to values shown by true solutions at same concentrations.
-
Tyndall effect is light scattering by colloidal particles. Tyndall effect is observed only when:
-
Color of the colloidal solution depends on the wavelength of the light that is scattered by the dispersed particles. Change in the particle size also changes the color of the colloidal particles.
-
Zig-zag movement of colloidal particles is known as Brownian Movement. Size of the particles and viscosity affects the Brownian movement.
-
Colloidal particles always carry some electric charge.
Emulsions
Liquid-liquid colloidal systems are known as emulsions. There are basically two types of emulsions:
-
Oil dispersed in water. In this, water acts as dispersion medium. For Example, milk and vanishing cream.
-
Water dispersed in oil. In this, oil acts as dispersion medium. For Example, butter and cream.
We will discuss the surface chemistry and its various related concepts under following sub topics:
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Click here to access the syllabus of chemistry for IIT JEE
To read more, Buy study materials of Surface Chemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
Watch this Video for more reference
More Readings