Fuel Cell
Table of Content |
Introduction to Fuel Cell
Sir William Grove invented first fuel cell in 1839. He just felt that when electricity can split water into Hydrogen and oxygen respectively, then by reversing this method can they produce opposite result.
They are those devices that efficiently and cleanly generate electricity with help of electrochemical process in which hydrogen and oxygen rich fuel comes together to form water and current.
In this type of cells hydrogen cation (H+) either react with oxygen or an oxidizing agent.
We can even use Methane, Ethane, Ethanol, Propanol, Gasoline etc. The major by product is water and some minor byproducts include nitrous oxide.
It’s different rather unique from batteries because cell need an unstoppable/continuous supply of fuel and air having oxygen to keep its chemical reaction alive and on. Whereas in case of batteries the chemicals inserted in it react continuously to generate electricity. In nutshell it can be said that fuel cell can keep on producing electricity as long as there reactants are supplied, but batteries get depleted after some time.
Fuel cell came into discovery arena in early 1840. For the very first time fuel cell was used commercially by NASA to launch space shuttles. Now a days fuel cells are used as power backup for industries, commercial housing areas even used in some vehicles, motorcycles, submarines, boats, including forklifts etc.
Types of Fuel Cells
|
Low Temperature Fuel Cells
|
1.Direct Methanol Fuel Cell (DMFC) |
|
2.Proton Exchanger (PEMFC) |
|
|
3. Alkaline Fuel Cell (AFC) |
|
High Temperature Fuel Cells |
1.Phosphoric Acid Fuel Cell (PAFC) |
|
2. Molten Carbon Fuel Cell (MCFC) |
|
|
3. Solid Oxide Fuel Cell (SOFC) |
|
|
4. Hydrogen-Oxygen Fuel Cell (HOFC) |
Note: They are classified on the basis of electrolyte used in it, DMFC is an exception to it. There are other forms of fuel cell even which is used in high level research.
Study of Hydrogen-Oxygen Fuel Cell
-
In this cell the fuel hydrogen is oxidized by air or oxygen to produce water and energy.
-
It’s a good alternative to rechargeable batteries and cells.
-
In this cell an exothermic reaction takes place, which release a lot of energy.
Energy Level Diagram of HOFC
Diagramatic Representation of HOFC
Animation of HOFC
Working of HOFC
At Anode:
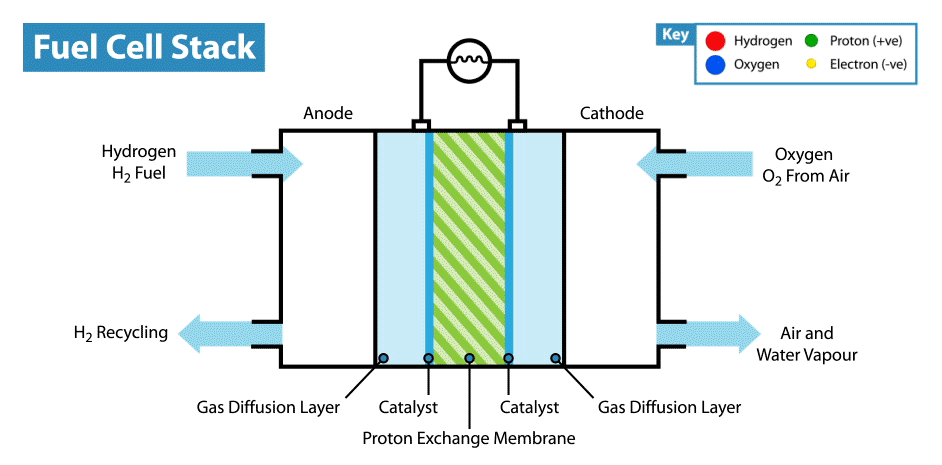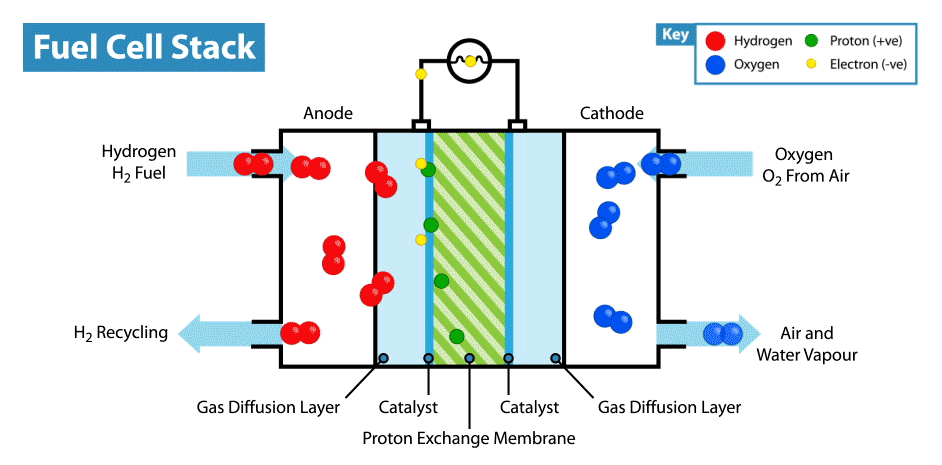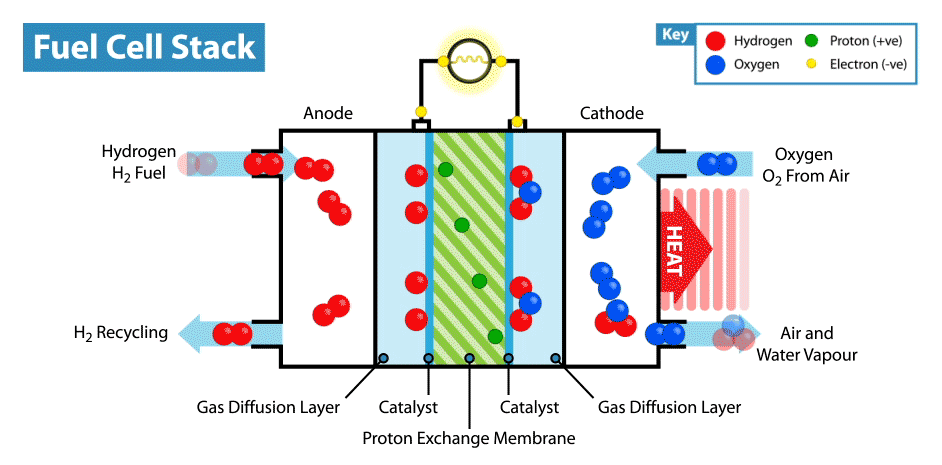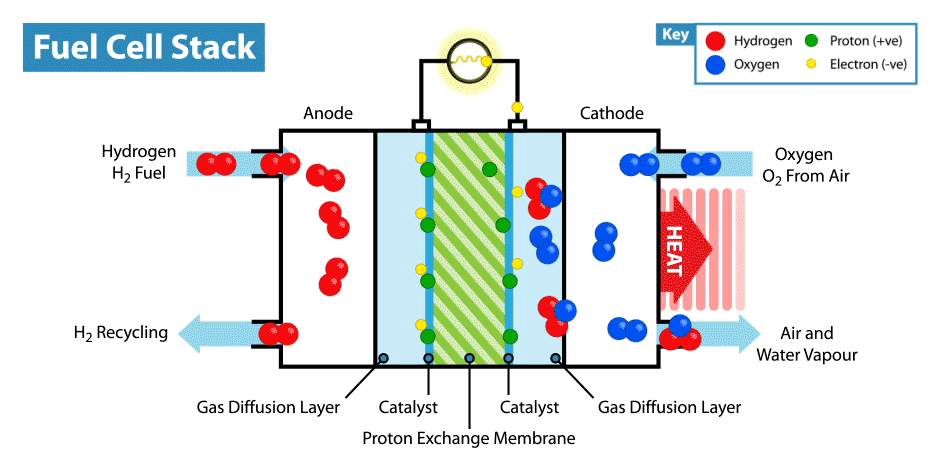
H2 gas added to the anode. Anode is layered with platinum which behaves as a catalyst which helps to covert hydrogen to hydrogen cations and free electrons. This free electron travels from anode to cathode through the connected complete circuit and supply power to the bulb/load linked to circuit because flow of electrons produce electricity.
Anodic reaction: 2H2 → 4H+ + 4e−
Since electrons are lost at anode it is known as Oxidation Reaction.
At Cathode:
The H+ ion generated from hydrogen gas at anode travels through electrolyte and reaches cathode. The oxygen which is received at cathode reacts with electrons coming in from anode side and H+ ion to generate some heat and water which is excreted out from the cell.
Cathodic Reaction: O2 + 4H+ + 4e− → 2H2O + Heat
Since electrons are gained at cathode it is known as Reduction Reaction.
Question. Can operation of vehicles possible by hydrogen-oxygen fuel cell?
Sol. Yes, it can be used to run vehicles that run on electricity.
The vehicles run on conservation of energy and convert energies from one form to another:
Chemical Energy to Thermal Energy to Mechanical Energy
Even the vehicles that run on fuel cell can covert energies like this:
Chemical Energy to Electrical Energy to Mechanical Energy
Advantages of HOFC
-
The reactant i.e. Hydrogen and oxygen is easily available.
-
It does not cause pollution.
-
Its environmental friendly.
-
It’s used as rocket fuel.
-
This fuel is highly efficient.
-
It’s a renewable form of energy.
-
Low temperature fuel cells can be used in military applications because it emits low heat.
-
It has large operation time than batteries therefore can save less time and money for a country.
Disadvantage of Fuel Cells
-
The method of production is expensive.
-
It is difficult to store.
-
It’s highly flammable.
-
It is dependent on fossil duel to separate it from nature.
-
It cannot hold more fuel at a single time.
-
It emit nitrogen dioxide.
Watch this Video for more reference
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More