IIT JEE Metallic and Hydrogen Bonding | JEE Chemical Bonding
Table of Content |
Metallic Bonding
 Metals are characterised by bright, lustre, high electrical and thermal conductivity, malleability, ductility and high tensile strength. A metallic crystal consists of very large number of atoms arranged in a regular pattern. Different model have been proposed to explain the nature of metallic bonding two most important modules are as follows
Metals are characterised by bright, lustre, high electrical and thermal conductivity, malleability, ductility and high tensile strength. A metallic crystal consists of very large number of atoms arranged in a regular pattern. Different model have been proposed to explain the nature of metallic bonding two most important modules are as follows
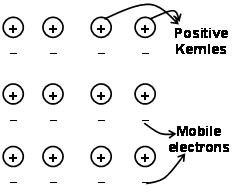
The forces that hold the atoms together in a metal as a result of the attraction between positive ions and surrounding freely mobile electrons are known as metallic bonds.
Through the electron sea predated quantum mechanics it still satisfactorily explains certain properties of the metals. The electrical and thermal conductivity of metals for example, can be explained by the presence of mobile electrons in metals. On applying an electron field, these mobile electrons conduct electricity throughout the metals from one end to other. Similarly, if one part of metal is heated, the mobile electrons in the part of the metals acquire a large amount of kinetic energy. Being free and mobile, these electrons move rapidly throughout the metal and conduct heat to the other part of the metal.
Refer to the following video for mettalic bonding
On the whole this model is not satisfactory.
Hydrogen Bonding
An atom of hydrogen linked covalently to a strongly electronegative atom can establish an extra weak attachment to another electronegative atom in the same or different molecules. This attachment is called a hydrogen bond. To distinguish from a normal covalent bond, a hydrogen bond is represented by a broken line eg X – H…Y where X & Y are two electronegative atoms. The strength of hydrogen bond is quite low about 2-10 kcal mol–1 or 8.4–42 kJ mol–1 as compared to a covalent bond strength 50–100 kcal mol–1 or 209 –419 kJ mol–1
Conditions for Hydrogen Bonding
Hydrogen should be linked to a highly electronegative element.
The size of the electronegative element must be small.
These two criteria are fulfilled by F, O, and N in the periodic table. Greater the electronegativity and smaller the size, the stronger is the hydrogen bond which is evident from the relative order of energies of hydrogen bonds.
Types of Hydrogen Bonding
Intermolecular hydrogen bonding:This type of bonding takes place between two molecules of the same or different types. For example,
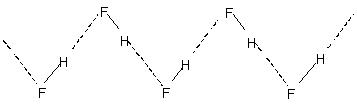
Intermolecular hydrogen bonding leads to molecular association in liquids like water etc. Thus in water only a few percent of the water molecules appear not to be hydrogen bonded even at 90°C. Breaking of those hydrogen bonds throughout the entire liquid requires appreciable heat energy. This is indicated in the relatively higher boiling points of hydrogen bonded liquids. Crystalline hydrogen fluoride consists of the polymer (HF)n. This has a zig-zag chain structure involving
H-bond.
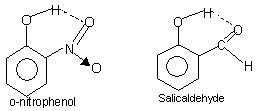
Intramolecular hydrogen bonding: This type of bonding occurs between atoms of the same molecule present on different sites. Intramolecular hydrogen bonding gives rise to a closed ring structure for which the term chelation is sometimes used. Examples are
o-nitrophenol, salicylaldehyde.
Importance of Hydrogen Bonding in Biological Systems
Hydrogen bonding plays a vital role in physiological systems. Proteins contain chains of amino acids. The amino acid units are arranged in a spiral form somewhat like a stretched coil spring (forming a helix). The N-H group of each amino acid unit and the fourth C=O group following it along the chain, establishes the N–H---O hydrogen bonds. These bonds are partly responsible for the stability of the spiral structure. Double helix structure of DNA also consists of two strands forming a double helix and are joined to each other through hydrogen bond.
Effect of Hydrogen Bonding
Hydrogen bonding has got a very pronounced effects on certain properties of the molecules. They have got effects on
-
State of the substance
-
Solubility of the substance
-
Boiling point
-
Acidity of different isomers
These can be evident from the following examples.
Example. H2O is a liquid at ordinary temperature while H2S is a gas although both O and S belong to the same group of the periodic table.
Solution: H2O is capable of forming intermolecular hydrogen bonds. This is possible due to high electronegativity and small size of oxygen. Due to intermolecular H-bonding, molecular association takes place. As a result the effective molecular weight increases and hence the boiling point increases. So H2O is a liquid. But in H2S no hydrogen bonding is possible due to large size and less electronegativity of S. So it’s boiling point is equal to that of an isolated H2S molecule and therefore it is a gas.
Example.Ethyl alcohol (C2H5OH) has got a higher boiling point than dimethyl ether (CH3-O-CH3) although the molecular weight of both are same.
Solution: Though ethyl alcohol and dimethyl ether have the same molecular weight but in ethyl alcohol the hydrogen of the O-H groups forms intermolecular hydrogen bonding with the OH group in another molecule. But in case of ether the hydrogen is linked to C is not so electronegative to encourage the hydrogen to from hydrogen bonding.
Due to intermolecular H-bonding, ethyl alcohol remains in the associated form and therefore boils at a higher temperature compared to dimethyl ether.
Related Resources
Reference books of Physical Chemistry
To read more, Buy study materials of Chemical Bonding comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More