IIT JEE Biomolecules | JEE Important Topics in Biomolecules
Table of Content |
Definition of Biomolecules
 A Biomolecule is any molecule present in living organisms including large macromolecules such as carbohydrates, proteins, nucleic acids & lipids, and the small molecules such as primary and secondary metabolites & natural products.
A Biomolecule is any molecule present in living organisms including large macromolecules such as carbohydrates, proteins, nucleic acids & lipids, and the small molecules such as primary and secondary metabolites & natural products.
What are 4 types of Biomolecules that make up the Living Organisms?
It includes proteins, lipids, carbohydrates and nucleic acids. Most of the biomolecules are composed of oxygen, hydrogen, nitrogen and carbon.
Proteins
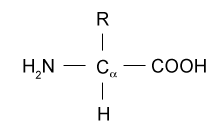
Proteins are biomolecules that are essential for the survival of the living organisms. Amino acids are the building blocks of the proteins. There are 22 naturally occurring amino acids. Amino acid is composed of amino group, carboxyl group, hydrogen atom and R group (alkyl group). The R group is variable, that is, varies with different amino acids. These 4 groups are attached to single carbon atom known as Alpha-Carbon.
Fig.1. Structure of Amino Acid
The simplest amino acid is glycine. In glycine, the R group is replaced by hydrogen atom. The bond between the two amino acid is peptide bond.
Fig.2. Amino Acid Sequence-Protein
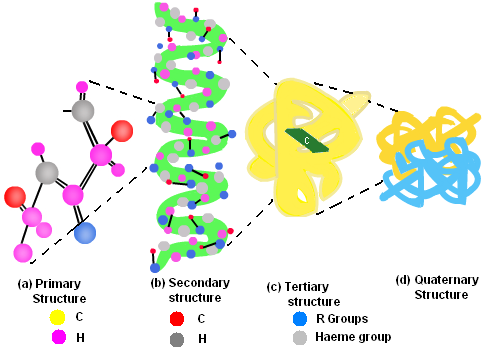
Structure of Protein
The protein exists most commonly in four different forms:
Primary Structure: The sequence of amino acids is called the primary structure of a protein. The left end is represented by the first amino acid, while the right end is represented by the last amino acid. The first amino acid is also called N-terminal amino acid. The last amino acid is called C-terminal amino acid.
Secondary Structure: The protein is not a linear chain of amino acids rather the chain would bend at some places and even form helices. Regularly repeating local structures gives secondary structure to protein.
Tertiary Structure: The overall shape of a protein molecule; and the spatial relationship of the secondary structures to one another; is called tertiary structure of protein. In other words, the various folds which give three dimensional appearances to protein form its tertiary structure.
Quaternary Structure: The manner in which the individual folded polypeptides are arranged with respect to each other is called quaternary structure of protein
What type of Biomolecules is an Enzyme?
Enzyme belongs to protein. All enzymes are protein except ribonucleases.
Lipids
Lipids are usually insoluble in water. They are fatty acid esters. They are the principal component of cell membranes. Lipids can be simple fatty acids and some lipids have phosphorous and phosphorylated organic compounds in them. Lipids, containing phosphorus; are called phospholipids. A fatty acid has a carboxyl group attached to an R group.
Fig.4. Structure of Phospholipids
The R group can be a methyl or ethyl or higher number of CH2 group (1 carbon to 19 carbons).
There are mainly two types of fatty acids- Saturated and unsaturated fatty acids.
Saturated fatty acids do not contain any double bond between the carbon atoms. For Example, Butyric acid.
Unsaturated fatty acids contain double bonds between the carbon atoms. For Example, Linoleic acid.
Carbohydrates
Carbohydrates are made up of carbon, hydrogen and oxygen atoms. It includes sugars, cellulose and starch. The simplest carbohydrate is glucose. The bond between two sugar molecule is known as glycosidic bond.
Fig.5. Structure of Glucose
Carbohydrates made up of single sugar molecule is known as monosaccharide. For Example, glucose. Monosaccharides made up of more than one unit of sugar molecule is known as oligosaccharides. For Example, fructose. The long chains of sugars are called polysachharides. If a polysaccharide is made up of similar monosaccharides, it is called Homopolymer, e.g. cellulose. If a polysaccharide is made up of different monosachharides, it is called heteropolymer.
The right end of a polysaccharide chain is called the reducing end and the left end is called the Non-Reducing end.
Fig.6. Reducing and Non-Reducing ends in Maltose
Starch is a homopolymer of glucose. It is the major storage sugar in plants. Similarly, in animals, the storage form of sugar is glycogen. This is stored in liver. When body needs glucose at the time of starvation or fast, glycogen breaks down into glucose to meet the energy requirement of the body. The sugar present in DNA is deoxyribose whereas in RNA, the sugar is ribose.
Nucleic Acids
Nucleic Acids are organic molecules present in living cells. Nucleic acids are polymers of nucleotides. There are three chemically distinct components in a nucleotide. These are as follows - Phosphate group, sugar known as deoxyribose and nitrogenous bases. There are two types of nitrogenous bases- Purines and Pyrimidines. Purines includes adenine and guanine whereas pyrimidines include thymine and cytosine. DNA contain all 4 bases, that is, adenine, guanine, thymine and cytosine. But in RNA, thymine is replaced by uracil.
What Type of Biomolecules is DNA?
DNA is a nucleic acid biomolecules.
Fig.6. Structure of Nitrogenous Bases
Important topic
You can also refer to
JEE Organic Chemistry Syllabus
Reference books of Organic Chemistry
To read more, Buy study materials of Biomolecules comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
Watch this Video for more reference