Imperfection in Solids
Table of contents |
Introduction to Imperfection in Solids
Any irregularity in the pattern of crystal arrangement in a solid lattice is called imperfection in solids. The occurrence of defects takes place when crystallization (the process of formation of crystals) occurs at a very fast or at an intermediate rate. This is because particles don’t get enough time to arrange themselves in a regular pattern.
Basically, defects fall out in two forms:
-
Point Defect
-
Line Defect
Point Defect
Point Defect occurs when an atom is missing or is irregularly arranged in a crystal lattice. In other words, the deviations and irregularities observed around an atom or a point are known as a Point Defect.
Types of Point Defects
Point Defects are classified into two defects, namely:
-
Stoichiometric Defects
-
Nonstoichiometric Defects
Stoichiometric Defects
The defects due to which the stoichiometry of solids remains undisturbed are called stoichiometric Defects. After stoichiometric defect, the ratio of cation and anions remain same in a solid. They are also known as intrinsic or thermodynamic defects.
Stoichiometric Defects are further classified into two defects:
-
Vacancy Defect
-
Interstitial Defect
-
Schottky Defect
-
Frenkel Defect
Image 2: Frenkel Defect
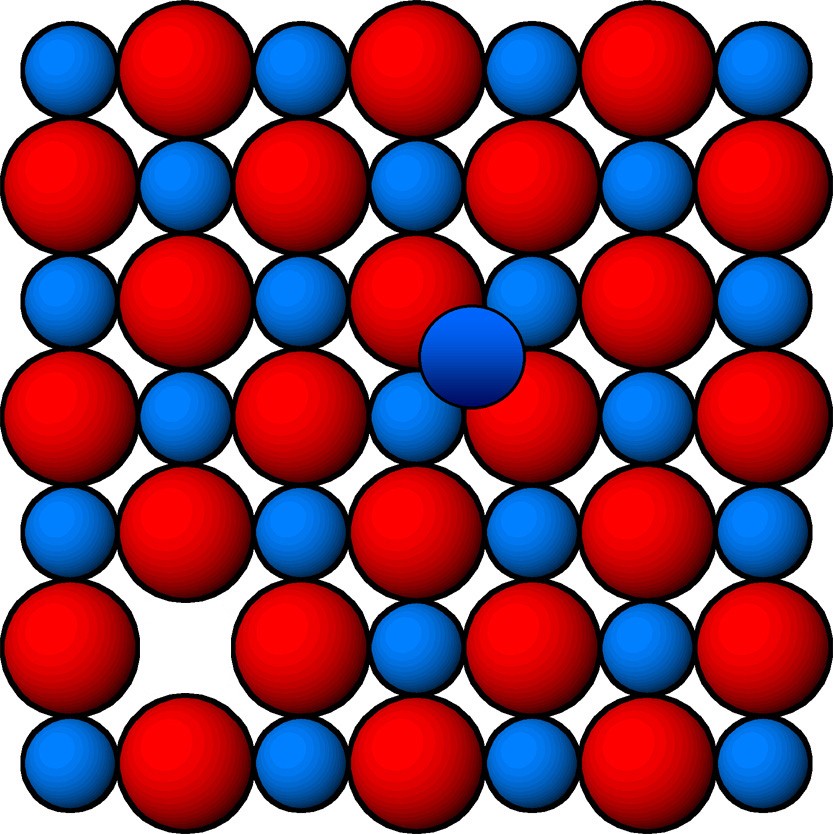
Vacancy Defect
Image 3: Vacancy Defect
Formation of vacancy defects take place due to vacant lattice sites. This defect generally occurs when we heat a solid. Vacancy defect also decreases the density of solid.
Interstitial Defect
Image 4: Interstitial Defect
When some extra constituent particles (atoms, molecules or ions) are acquainted in the interstitial sites, the solid lattice is said to have an interstitial defect. Interstitial Defects are responsible for increasing density of the substance.
Both vacancy and interstitial defects are depicted by non-ionic solids whereas in ionic solids these defects occur in form of Schottky and Frenkel Defect. Remember electrical neutrality is of utmost importance for the existence of ionic solids.
Schottky Defect
If in an ionic lattice equal number of cations and anions are missing from the lattice site, the lattice is said to have Schottky defect. In Schottky Defect the electrical neutrality is maintained and it generally takes place in ionic solids. The compounds in which the size of cation and anion are nearly equal tend to show this type of defect. Some common examples of compounds depicting Schottky Defect are:
-
Sodium Chloride ( NaCl)
-
Potassium Chloride ( KCl)
-
Cesium Chloride ( CsCl)
-
Silver Bromide ( AgBr)
Schottky Defect also decreases the density of the crystal lattice.
Image 5: Schottky Defect
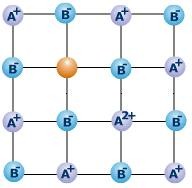
Frenkel Defect
Just like Schottky defect Frenkel defect is also observed in ionic solids. When a smaller ion (generally cation) is moved from its normal position to a new interstitial site, Frenkel Defect occurs. This dislocation of ion creates vacancy defect at its original site and interstitial defect at its new position. The density of the crystal lattice remains same in Frenkel Defect. Ionic solids in which there are vast size differences between cation and ion tend to depict Frenkel Defect. Some common examples of compounds depicting Frenkel Defect are as follows:
-
Zinc Sulphide ( ZnS)
-
Silver Chloride ( AgCl)
-
Silver Bromide ( AgBr)
-
Silver Iodide ( AgI)
Non-Stoichiometric Defect
The defect in which the ratio of cation and anion in an ionic lattice is variable is known as a non-stoichiometric defect. They are of two types, namely:
-
Metal Excess Defect
-
Metal Deficiency Defect
Metal Excess Defect
Metal Excess Defect occurs in two ways:
-
Due to anionic vacancies
-
Due to presence of extra cations at interstitial sites
Due to anionic vacancies
Metal Excess Defect can be seen in alkali halides like NaCl and KCl. In this type of defect, the anionic vacancies are missing and are filled by an electron to ensure electrical neutrality. For Example, when a NaCl crystal is heated in presence of sodium vapor, the sodium atoms are deposited on the surface of the metal. The Cl- ions diffuse out from the crystal surface and combines with Na atom to give NaCl. To make this happen sodium loses electrons to become Na+ and the released electrons occupy the anionic vacancies. As a result, crystal gets excess sodium.
The anionic sites or vacancies which are filled by unpaired electrons are called F-centers and are responsible for imparting a yellow color to the flame of NaCl. Similarly in KCl excess of potassium is responsible for making KCl crystal flame violet.
Due to presence of extra cations at interstitial sites
In this type of defect, extra positive ions and electrons occupy interstitial sites and maintain electrical neutrality. In other words, this defect is losing non-metal atoms leaving their electrons behind and excess metal occupies interstitial positions. For Example, in white zinc oxide on heating, it leaves oxygen and becomes yellow.
ZnO → Zn+2 + O2 + 2e-
Now due to excess of zinc in the crystal, the formula of ZnO becomes Zn1+xO.
Metal Deficiency Defect
This defect occurs in metals containing less number of cations than anions. It is mostly seen in compounds depicting variable oxidation states. Due to unequal number of cations and anions the metals obtained are non-stoichiometric. Some common examples falling under this category of defect are Fe0.95O, Fe0.93O etc.
Image 6: Metal Deficiency Defect
Watch this Video for more reference
More Readings