Samyak Jain
Last Activity: 5 Years ago
RMS speed of ideal gas molecules is
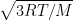
, where T is the temperature in kelvin, R is universal gas constant,
M is the molar mass of gas.
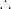
ratio of rms speeds of molecules at T
2 and T
1 is
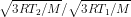
=
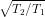
…..(1)
Now, we need to compute temperatures T1 and T2.
We know that for isotherm, PV = constant i.e. PV = nRT and T is constant.
Consider isotherm at T1. It is passing through P = 1.105 Pa and V = 1 m3.
So, 1.105 Pa . 1 m3 = nR T1 or T1 = (105 / nR) K
Similarly, for T2, P = 2.105 Pa and V = 2 m3.
So, 2.105 Pa . 2 m3 = nR T2 or T2 = (4.105 / nR) K
Substitute value of T1 and T2 in (1) and you will get
ratio of rms speeds =
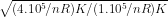
=
2 .





