Samyak Jain
Last Activity: 6 Years ago
Efficiency of Carnot heat engine depends on temperatures of source and sink and also depends on working substance.
Efficiency of Carnot cycle,
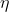
= (Work done) / (Heat supplied) = W / q
1From first law of thermodynamics, q =
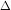
U + W.
Since Carnot cycle is a cyclic process,
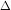
U = 0 i.e. q = W = Heat absorbed by system = q
1 – q
2where q2 = heat given out to the sink.

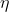
= (q
1 – q
2)/ q
1 = 1 – q
2/q
1 .
Also, 1 – T2 / T1.
Hence, we can say that efficiency of Carnot heat engine is independent of thermal capacity of source or sink.





