Kapil Khare
Last Activity: 6 Years ago
Let us assume the final temperature to be T.
Now, we also know that T should be less than 25

C and greater than -14

C. Let us initially assume that our final state is water i.e. T is greater than 0

C. If after solving we get T less than 0

C then, we will say that our assumption is wrong and the final state is ice.
Magnitude of heat released by water to reach the equlibrium temperature = (200)(1)(25-T) calories
Magnitude of heat aborbed by the ice to reach 0

C = (30)(14)(0.5) calories = 210 calories
Magnitude of heat aborbed by the ice to meat to water = (30)(80) calories = 2400 calories
Magnitude of heat absorbed by this water to reach temperature T from 0

C = (30)(T-0)(1) calories
magnitude of total heat absorbed = (2610 + 30T) calories
magnitude of total heat absorbed = magnitude of total haet released
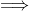
2610 + 30T = 5000 – 200T
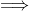
230T = 2390
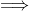
T = 10.39

C










