Dear sowlabhya ramapriya,
For a reaction at constant temperature and pressure, ΔG in the Gibbs free energy is:
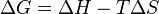
When ΔG is negative, a process or chemical reaction proceeds spontaneously in the forward direction.
When ΔG is positive, the process proceeds spontaneously in reverse.
When ΔG is zero, the process is already in equilibrium, with no net change taking place over time.
Hope this was helpful..!!
Please do not forget to give me a Thumbs Up by approving my answer by clicking on "Yes" below..!!!




