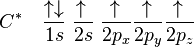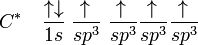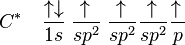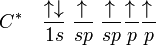AKASH GOYAL AskiitiansExpert-IITD
Last Activity: 14 Years ago
Dear Kedar
It is the imaginary mixing of the 2s, 2px, 2py and 2pz atomic orbitals of carbon to form a new set of 'hybrid' orbitals that orient themselves in the desired VSEPR geometry. The hybrid orbitals are equivalent to one another making all orbital overlaps equivalent, therefore, all C-H bonding interactions equivalent.

Hybrid orbitals are named by considering the type and number of atomic orbitals from which they arose. For CH4 then the hybridisation for the carbon is sp3
Types of Hybridisations
sp3 hybrids
The first step in hybridisation is the excitation of one (or more) electrons (we consider the carbon atom in methane, for simplicity of the discussion):
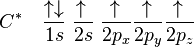
The proton that forms the nucleus of a hydrogen atom attracts one of the lower-energy valence electrons on carbon. This causes an excitation, moving a 2s electron into a 2p orbital.
In the case of carbon attempting to bond with four hydrogens, four orbitals are required. Therefore, the 2s orbital (core orbitals are almost never involved in bonding) "mixes" with the three 2p orbitals to form four sp3 hybrids (read as s-p-three). See graphical summary below.
becomes 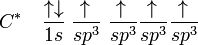
In CH4, four sp3 hybridised orbitals are overlapped by hydrogen's 1s orbital, yielding four σ (sigma) bonds (that is, four single covalent bonds). The four bonds are of the same length and strength. This theory fits our requirements.
 translates into
translates into 
An alternative view is: View the carbon as the C4− anion. In this case all the orbitals on the carbon are filled:

If we now recombine these orbitals with the empty s-orbitals of 4 hydrogens (4 protons, H+) and allow maximum separation between the 4 hydrogens (i.e., tetrahedral surrounding of the carbon), we see that at any orientation of the p-orbitals, a single hydrogen has an overlap of 25% with the s-orbital of the C, and a total of 75% of overlap with the 3 p-orbitals (see that the relative percentages are the same as the character of the respective orbital in an sp3-hybridisation model, 25% s- and 75% p-character).
sp2 hybrids
Other carbon based compounds and other molecules may be explained in a similar way as methane. Take, for example, ethene (C2H4). Ethene has a double bond between the carbons
For this molecule, carbon will sp2 hybridise, because one π (pi) bond is required for the double bond between the carbons, and only three σ bonds are formed per carbon atom. In sp2 hybridisation the 2s orbital is mixed with only two of the three available 2p orbitals:
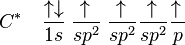
forming a total of 3 sp2 orbitals with one p-orbital remaining. In ethylene (ethene) the two carbon atoms form a σ bond by overlapping two sp2 orbitals and each carbon atom forms two covalent bonds with hydrogen by s–sp2 overlap all with 120° angles. The π bond between the carbon atoms perpendicular to the molecular plane is formed by 2p–2p overlap. The hydrogen-carbon bonds are all of equal strength and length, which agrees with experimental data
sp hybrids
The chemical bonding in compounds such as alkynes with triple bonds is explained by sp hybridization.
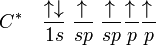
In this model, the 2s orbital mixes with only one of the three p-orbitals resulting in two sp orbitals and two remaining unchanged p orbitals. The chemical bonding in acetylene (ethyne) (C2H2) consists of sp–sp overlap between the two carbon atoms forming a σ bond and two additional π bonds formed by p–p overlap. Each carbon also bonds to hydrogen in a sigma s–sp overlap at 180° angles.
All the best.
AKASH GOYAL
AskiitiansExpert-IITD
Please feel free to post as many doubts on our discussion forum as you can. We are all IITians and here to help you in your IIT JEE preparation.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar..