Dear Sachin,
According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made.
Suppose you have an equilibrium established between four substances A, B, C and D.

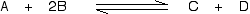
According to Le Chatelier's Principle, if you decrease the concentration of C, for example, the position of equilibrium will move to the right to increase the concentration again.
Please feel free to ask your queries here. We are all IITians and here to help you in your IIT JEE preparation.
All the best.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar..
Askiitians Expert
Anil Pannikar
IIT Bombay




