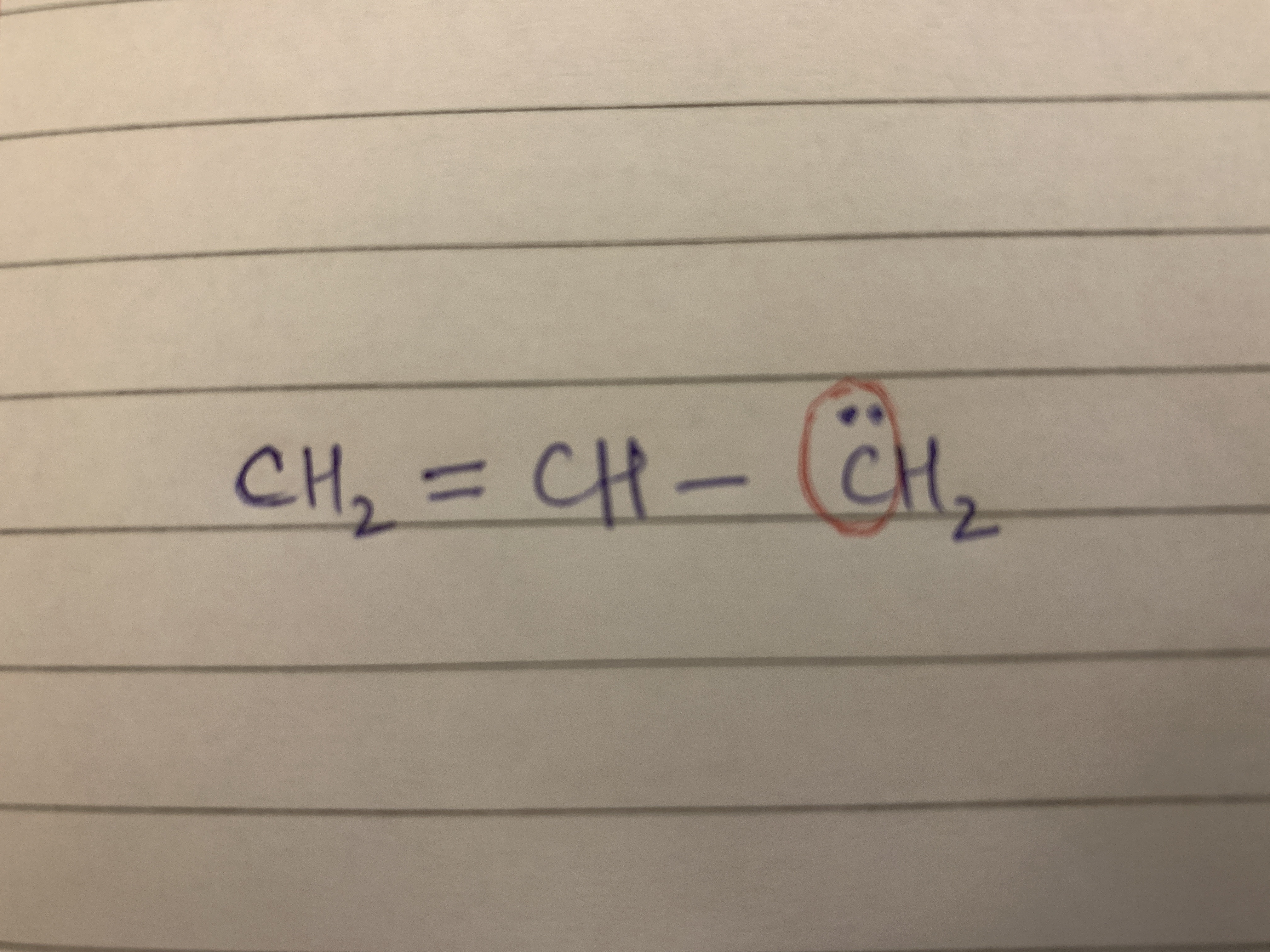What are pure p orbitals. Please explain in detail with an example. In the attachment, how is the carbon atom with the lone pair sp2 hybridised and not sp3?
What are pure p orbitals. Please explain in detail with an example. In the attachment, how is the carbon atom with the lone pair sp2 hybridised and not sp3?