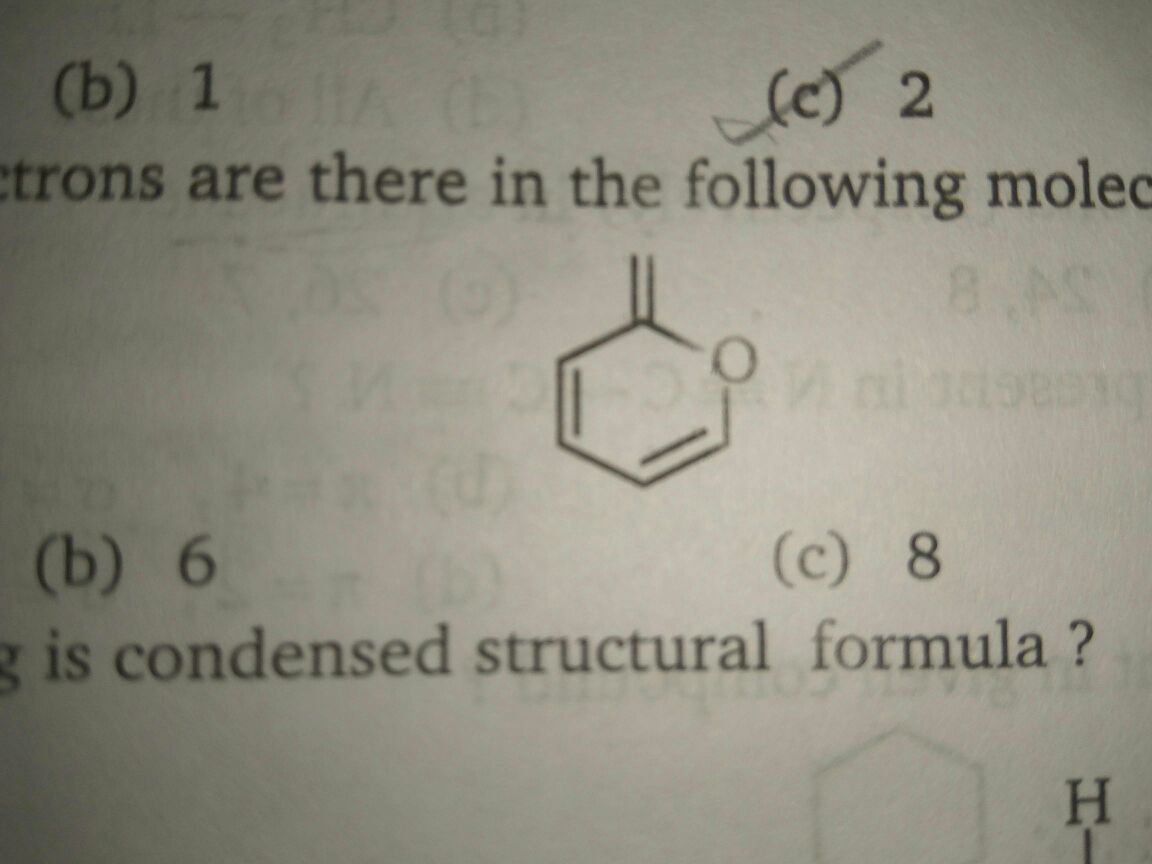
(I posted this question many time but I not found any satisfactory answer.Please ,sir help me.This is a question of MS Chouhan .Please expalin in detail why we consider both the lone pairs on oxygen as it has only 1 pi orbital so it must have only 1 pair of pi electron after resonance also.Please explain in detail as I fed up after posting this same question many timesQ-Calculate the number of pi electrons present in the following compound(see attachment)?Ans-10
(I posted this question many time but I not found any satisfactory answer.Please ,sir help me.This is a question of MS Chouhan .Please expalin in detail why we consider both the lone pairs on oxygen as it has only 1 pi orbital so it must have only 1 pair of pi electron after resonance also.Please explain in detail as I fed up after posting this same question many times
Q-Calculate the number of pi electrons present in the following compound(see attachment)?
Ans-10