How do decide whether the electron density on a ring is greater or less in the presence of certain functional groups.
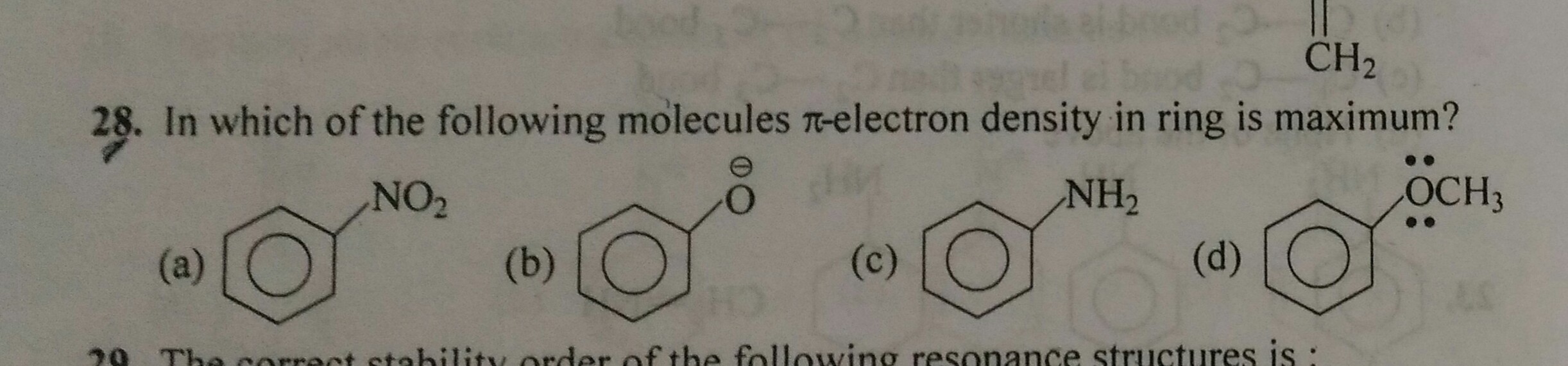

Last Activity: 3 Years ago

Last Activity: 3 Years ago

Last Activity: 3 Years ago

Last Activity: 3 Years ago