the above order is in aqueous solution. In aqueous solution in addition to inductive effect of alkyl group, stability of protonated ion (i.e.
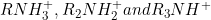
) due to H bonding with solvent. Thus, accounting both factors (+I of alkyl group and stability of protonated ion, it has been observed that secondary amine is clear winner, in case of primary and tertiary one, there is minor difference in the basicity order with tertiary slightly better than primary when alkyl group is ethyl (not in case of methyl group primary is more basic than tertiary).




