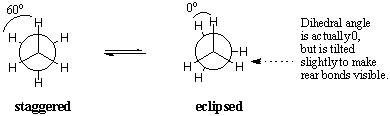SAGAR SINGH - IIT DELHI
Last Activity: 15 Years ago
Dear anurag,
Conformations of Ethane
While there are an infinite number of conformations about any sigma bond, in ethane two particular conformers are noteworthy and have special names. In the eclipsed conformation, the C-H bonds on the front and back carbons are aligned with each other with dihedral angles of 0 degrees. In the staggered conformation, the C-H bonds on the rear carbon lie between those on the front carbon with dihedral angles of 60 degrees.
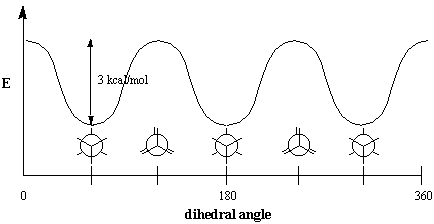
Energetically, not all conformations are equally favored. The eclipsed conformation of ethane is less stable than the staggered conformation by 3 kcal/mol. The staggered conformation is the most stable of all possible conformations of ethane, since the angles between C-H bonds on the front and rear carbons are maximized at 60 degrees. In the eclipsed form, the electron densities on the C-H bonds are closer together than they are in the staggered form. When two C-H bonds are brought into a dihedral angle of zero degrees, their electron clouds experience repulsion, which raises the energy of the molecule. The eclipsed conformation of ethane has three such C-H eclipsing interactions, so we can infer that each eclipsed C-H "costs" roughly 1 kcal/mol.

Figure %: Eclipsing interactions in ethane.
Steric Hindrance
Eclipsing interactions are an example of a general phenomenon called steric hindrance, which occurs whenever bulky portions of a molecule repel other molecules or other parts of the same molecule. Because such hindrance causes resistance to rotation, it is also called torsional strain. The 3 kcal/mol needed to overcome this resistance is the torsional energy. Note that this figure is very small compared to the energy required to rotate around double bonds, which is 60 kcal/mol (the bond energy of a C-C $\pi$ bond). At room temperature, ethane molecules have enough energy to be in a constant state of rotation. Because of this rapid rotation, it is impossible to isolate any particular conformation in the way that cis- and trans- alkenes can be individually isolated. Although the term "conformational isomer" is sometimes used as a synonym for conformations, conformations of a molecule are not considered true isomers because of their rapid interconversion.
We are all IITians and here to help you in your IIT JEE preparation.
All the best.
If you like this answer please approve it....
win exciting gifts by answering the questions on Discussion Forum
Sagar Singh
B.Tech IIT Delhi
sagarsingh24.iitd@gmail.com