If electrons were money, carbocations would be the beggars of organic chemistry. Packing a mere six valence electrons, these electron-deficient intermediates figure prominently in many reactions we meet in organic chemistry, such as
- nucleophilic substitution (SN1) and elimination (E1) reactions
- additions of electrophiles to double and triple bonds
- ,electrophilic aromatic substitution
- additions to carbonyl compounds and enolate chemistry (albeit in masked form)
That’s a huge chunk of sophomore O-chem, right there.
Being electron-deficient (and therefore unstable), formation of a carbocation is usually the rate-limiting step in these reactions.
Knowing that, then think about this: what happens to the rate of the reaction when the carbocation intermediate is made more stable? Well, the energy of the transition state leading to the reaction will be lower.
What’s that going to do to the rate of the reaction? It’s going to speed it up.
So what are some of the factors that stabilize carbocations?
If you look through all of your organic chemistry textbook, you’ll find 3 main structural factors that help to stabilize carbocations.
- Neighboring carbon atoms.
- Neighboring carbon-carbon multiple bonds
- Neighboring atoms with lone pairs.
Why is this? It all goes back to the core governing force in chemistry: electrostatics. Since “opposite charges attract, like charges repel”, you would be right in thinking that carbocations are stabilized by nearby electron-donating groups.
Let’s look at each of these in turn.
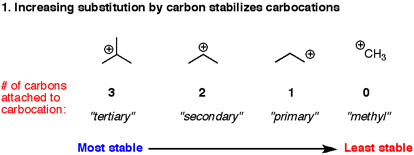
1) Carbocations are stabilized by neighboring carbon atoms.
The stability of carbocations increases as we go from primary to secondary to tertiary carbons. There’s two answers as to why this is. The age-old answer that is still passed around in many introductory textbooks points to carbons (alkyl groups in particular) as being “electron-releasing” groups through inductive effects. That is, a carbon (electronegativity 2.5) connected to hydrogen (electronegativity 2.2) will be electron rich, and can donate some of those electrons to the neighboring carbocation. In other words, the neighboring carbon pays the carbocation with electrons it steals from the hydrogens. The second, (and theoretically more satisfactory explanation) is hyperconjugation, which invokes stabilization through donation of the electrons in C-H sigma bonds to the empty p orbital of the carbocation.
Whatever the explanation, this factor governs many key reactions you meet in Org 1 – fromMarkovnikoff’s rule, to carbocation rearrangements, through understanding the SN1 and E1 reactions.
2) Carbocations are stabilized by neighboring carbon-carbon multiple bonds. Carbocations adjacent to another carbon-carbon double or triple bond have special stability because overlap between the empty p orbital of the carbocation with the p orbitals of the π bond allows for charge to be shared between multiple atoms. This effect, called “delocalization” is illustrated by drawing resonance structures where the charge “moves” from atom to atom. This is such a stabilizing influence that even primary carbocations – normally very unstable – are remarkably easy to form when adjacent to a double bond, so much so that they will actually participate in SN1 reactions.

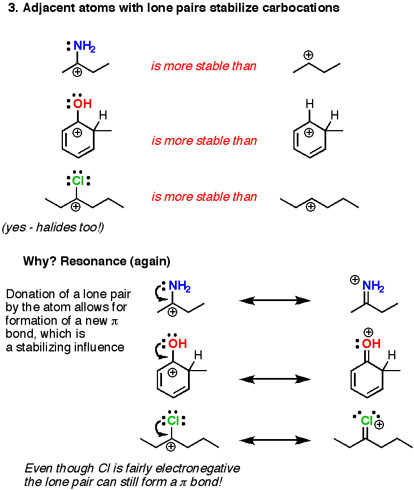
3) Carbocations are stabilized by adjacent lone pairs. The key stabilizing influence is a neighboring atom that donates a pair of electrons to the electron-poor carbocation. Note here that this invariably results in forming a double bond (π bond) and the charge will move to the atom donating the electron pair. Hence this often goes by the name of “π donation”.
The strength of this effect varies with basicity, so nitrogen and oxygen are the most powerful π donors. Strangely enough, even halogens can help to stabilize carbocations through donation of a lone pair. The fact that atoms that we normally think of as electron-wthdrawing (nitrogen, oxygen, chlorine) can actually be electron-donor groups is probably one of the most difficult factors to wrap your head around in Org 2.
This effect is tremendously important in the reactions of aromatic rings and also in enolate chemistry, where double bonds attached to donating groups (nitrogen and oxygen in particular) can be millions (or billions) of times more nucleophilic than alkenes that lack these groups.
The bottom line of this post is that by understanding the factors which affect the stability of carbocations, you can gain tremendous insight into many different reactions, even though they may appear vastly different.
Why is this important? Many reactions pass through carbocation intermediates. What do you think the effect of stabilizing the carbocation will be on the reaction rates? Here’s some specific examples.

PLEASE APPROVE MY ANSWER IF YOU LIKE IT
AND BEST OF LUCK!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!

















