Dear Gopal,
The role of the hydroxide ion in a substitution reaction
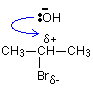
In the substitution reaction between a halogenoalkane and OH- ions, the hydroxide ions are acting as nucleophiles. For example, one of the lone pairs on the oxygen can attack the slightly positive carbon. This leads on to the loss of the bromine as a bromide ion, and the -OH group becoming attached in its place.
The role of the hydroxide ion in an elimination reaction
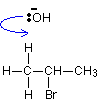
Hydroxide ions have a very strong tendency to combine with hydrogen ions to make water - in other words, the OH- ion is a very strong base. In an elimination reaction, the hydroxide ion hits one of the hydrogen atoms in the CH3group and pulls it off. This leads to a cascade of electron pair movements resulting in the formation of a carbon-carbon double bond, and the loss of the bromine as Br-.
Best Of luck
Cracking IIT just got more exciting,It s not just all about getting assistance from IITians, alongside Target Achievement and Rewards play an important role. ASKIITIANS has it all for you, wherein you get assistance only from IITians for your preparation and win by answering queries in the discussion forums. Reward points 5 + 15 for all those who upload their pic and download the ASKIITIANS Toolbar, just a simple to download the toolbar….
So start the brain storming…. become a leader with Elite Expert League ASKIITIANS
Thanks
Aman Bansal
Askiitian Expert



