vikas askiitian expert
Last Activity: 14 Years ago
Consider the two possible valence structures A and B for benzene:
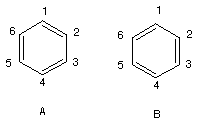
Note that both structures have the same number and kinds of bonds and therefore must be equal in energy.
-
- The difference is that in A, the double bonds are between C1-C2, C3-C4, and C5-C6, whereas in B the double bond positions are C2-C3, C4-C5, and C6-C1.
- In the real molecule benzene, none of the bonds are double bonds and none are single, all six bonds are equivalent and are intermediate between double and single (MO calculations indicate a bond order of 1.67).
- In the real molecule benzene, the bonds which are formally double in A and B do not really behave chemically like double bonds, but as a much more stable and less reactive functionality.
- This is because all six of the pi electrons in benzene are delocalized over all six atoms and are not uniquely shared by a given pair of two atoms.
In order to be able to continue to use our system of writing valence structures for molecules, we must adapt the system so that highly delocalized molecules like benzene can be realistically treated. Thus, no single valence structure gives a valid representation of benzene. In using resonance theory to adapt our structural representations to more accurately represent resonance stabilized molecules, we often need to represent such molecules by more than one valence structure:
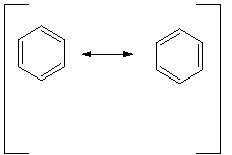
These valence structures are then called "resonance structures" or "canonical structures", because individually they do not adequately represent the structure of the real compound.As shown in the depiction below, the relationship between the two structures is usually shown by a curved arrow, which depicts the flow of electrons. Bear in mind, that in generating resonance structures, only electrons, not nuclei , are moved. Either electrons in bonds (usually pi bonds) or on atoms may be moved using the curved arrows.
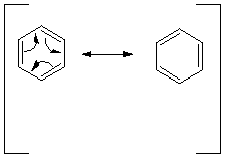
Note that the electrons that are moved in benzene are the electrons of the pi bond (called pi electrons).
CONVENTIONS FOR WRITING RESONANCE STRUCTURES:
-
- Write the first canonical structure, conforming to all the rules of valence.
- Use curved electron flow arrows to generate other structures.
- Connect the structures by double-headed arrows to indicate resonance , not equilibrium.
- Enclose the structures in brackets.



