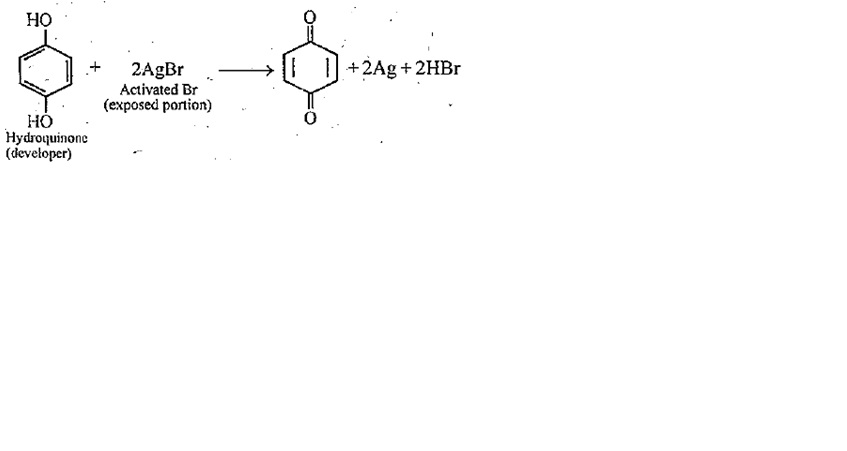
Write the chemical reaction involved in developing of a black and white photographic film. An aqueous Na2S2O3 solution is acidified to give a milky white tubitity. Identify the product and write the balanced half chemical reaction for it.
Write the chemical reaction involved in developing of a black and white photographic film. An aqueous Na2S2O3 solution is acidified to give a milky white tubitity. Identify the product and write the balanced half chemical reaction for it.