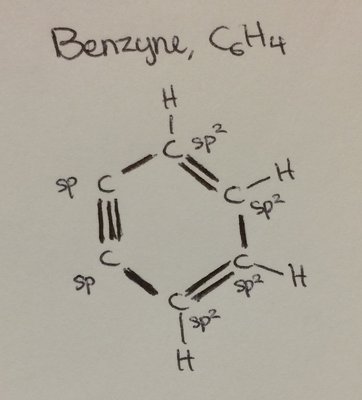
Why the Carbon atom at triple bond position in Benzyne not sp hybridized?Why are they sp2 hybridized in place of sp?
Why the Carbon atom at triple bond position in Benzyne not sp hybridized?Why are they sp2 hybridized in place of sp?

Last Activity: 3 Years ago

Last Activity: 3 Years ago

Last Activity: 3 Years ago


Last Activity: 3 Years ago