ratio of specific heat at a const. pressure and volume for hydrogen (Cp/Cv)=1.40. This indicates it is diatomic.
How did we come to this conclusion? How do we find out the atomicity for other elements if the ratio of specific heat is given?
ratio of specific heat at a const. pressure and volume for hydrogen (Cp/Cv)=1.40. This indicates it is diatomic.
How did we come to this conclusion? How do we find out the atomicity for other elements if the ratio of specific heat is given?





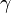 ) for an ideal gas can be related to the degrees of freedom (
) for an ideal gas can be related to the degrees of freedom ( 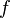 ) of a molecule by:
) of a molecule by:
 ,
,





