Dear Sangeetha,
1) The electron pairs in the valence shell around the central atom of a molecule repel each other and tend to orient in space so as to minimize the repulsions and maximize the distance between them.
2) There are two types of valence shell electron pairs viz., i) Bond pairs and ii) Lone pairs
Bond pairs are shared by two atoms and are attracted by two nuclei. Hence they occupy less space and cause less repulsion.
Lone pairs are not involved in bond formation and are in attraction with only one nucleus. Hence they occupy more space. As a result, the lone pairs cause more repulsion.
The order of repulsion between different types of electron pairs is as follows:
Lone pair - Lone pair > Lone Pair - Bond pair > Bond pair - Bond pair
Note: The bond pairs are usually represented by a solid line, whereas the lone pairs are represented by a lobe with two electrons.
3) In VSEPR theory, the multiple bonds are treated as if they were single bonds. The electron pairs in multiple bonds are treated collectively as a single super pair.
The repulsion caused by bonds increases with increase in the number of bonded pairs between two atoms i.e., a triple bond causes more repulsion than a double bond which in turn causes more repulsion than a single bond.
4) The shape of a molecule can be predicted from the number and type of valence shell electron pairs around the central atom.
When the valence shell of central atom contains only bond pairs, the molecule assumes symmetrical geometry due to even repulsions between them.
However the symmetry is distorted when there are also lone pairs along with bond pairs due to uneven repulsion forces.
5) Primary & Secondary effects on bond angle and shape:
i) The bond angle decreases due to the presence of lone pairs, which cause more repulsion on the bond pairs and as a result the bond pairs tend to come closer.
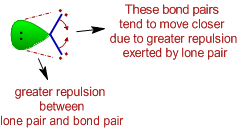
ii) The repulsion between electron pairs increases with increase in electronegativity of central atom and hence the bond angle increases. The bond pairs are closer and thus by shortening the distance between them, which in turn increases the repulsion. Hence the bonds tend to move away from each other.
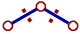 |
The bond pairs tend to move away from each other since the distance between them is shortened as they are more localized on more electronegative central atom. |
However the bond angle decreases when the electronegativities of ligand atoms are more than that of central atom. There is increase in the distance between bond pairs since they are now closer to ligand atoms. Due to this, they tend to move closer resulting in the decrease in bond angle.
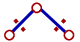 |
The bond pairs tend to come closer since the distance between them is increased as they are more localized on more electronegative ligand atoms. |
iii) The bond angle decreases with increase in the size of central atom.
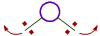 |
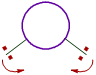 |
| On smaller central atoms the bond pairs are closer and hence tend to move away from each other so as to minimize repulsion. Hence bond angle will be more. |
On bigger central atoms, the bond pairs are more distant from each other and hence there is less repulsion. Hence they tend to move closer and thus by decreasing the bond angle. |
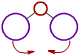
However the bond angle increases with increase in the size of ligand atoms, which surround the central atom.
 |
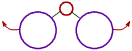 |
| There is less repulsion between smaller ligand atoms and they can move closer to each other and thus decrease the bond angle. |
These is more repulsion between bigger ligand atoms and hence they tend to move away from each other. Thus bond angle increases. |
iv) The bond angles are also changed when multiple bonds are present. It is due to uneven repulsions.
6) When there are two or more resonance structures, the VSEPR theory is applicable to any of such contributing structure.
Cracking IIT just got more exciting,It s not just all about getting assistance from IITians, alongside Target Achievement and Rewards play an important role. ASKIITIANS has it all for you, wherein you get assistance only from IITians for your preparation and win by answering queries in the discussion forums.
https://www.askiitians.com/packages/packages.aspx
So start the brain storming…. become a leader with Elite Expert League ASKIITIANS
Thanks
Aman Bansal
Askiitian Expert




