Dear student,
The term oxidation was originally used to describe reactions in which an element combines with oxygen.
Example: The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium.
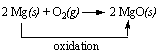
The term reduction comes from the Latin stem meaning "to lead back." Anything that that leads back to magnesium metal therefore involves reduction.
The reaction between magnesium oxide and carbon at 2000C to form magnesium metal and carbon monoxide is an example of the reduction of magnesium oxide to magnesium metal.

After electrons were discovered, chemists became convinced that oxidation-reduction reactions involved the transfer of electrons from one atom to another. From this perspective, the reaction between magnesium and oxygen is written as follows.
2 Mg + O2 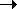 2 [Mg2+][O2-]
2 [Mg2+][O2-]
In the course of this reaction, each magnesium atom loses two electrons to form an Mg2+ ion.
Mg 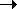 Mg2+ + 2 e-
Mg2+ + 2 e-
And, each O2 molecule gains four electrons to form a pair of O2- ions.
O2 + 4 e- 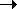 2 O2-
2 O2-
Please feel free to ask your queries here. We are all IITians and here to help you in your IIT JEE preparation.
All the best.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar..
Askiitians Expert
Sagar Singh
B.Tech, IIT Delhi




